Decitabine 5'-O-amino-acid ester prodrug and preparation method thereof
A technology of decitabine and ester prodrugs, which is applied in the field of medicine, can solve the problems of poor permeability of the small intestinal membrane, and achieve the effect of improving membrane permeability and oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
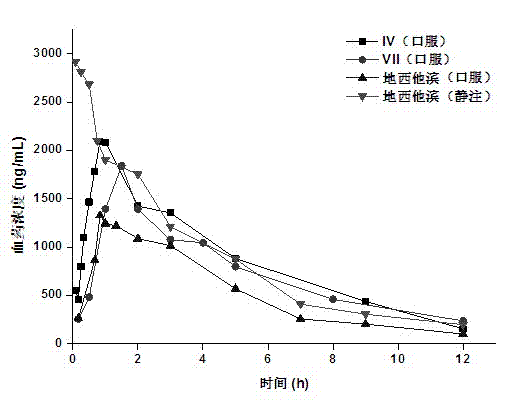
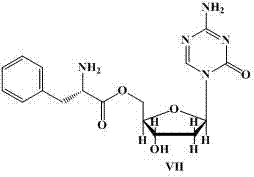
Image
Examples
Embodiment 1
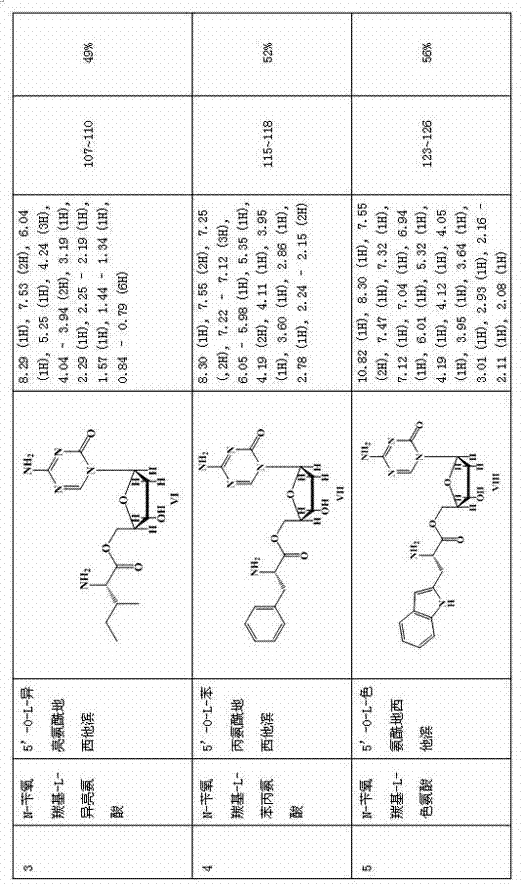
[0033] N-benzyloxycarbonyl-L-valine reacts with dicyclohexylcarbodiimide, the reaction solvent is anhydrous tetrahydrofuran, dichloromethane or N,N-4-dimethylformamide, and the reaction temperature is from 0°C to 80°C, preferably 0°C-50°C, the reaction time is 1 hour; the reaction solution is slowly added dropwise to the mixture of decitabine and N,N-4-dimethylaminopyridine. After the addition is complete, continue to react for 12 hours at a temperature of 0°C-40°C, preferably 10-30°C; Wash with distilled water, saturated sodium bicarbonate and saturated saline, collect the organic layer, dry the column with sodium persulfate, evaporate the filtrate to dryness, add Pd / C as a catalyst under the solvent of ethyl acetate, dichloromethane or isopropanol, and Catalytic hydrogenation was carried out under hydrogen conditions, the reaction time was 6 hours, suction filtration, and the filtrate was evaporated under reduced pressure to obtain the compound ( ).
Embodiment 2
[0035] N-benzyloxycarbonyl-D-valine reacts with dicyclohexylcarbodiimide, the reaction solvent is anhydrous tetrahydrofuran, dichloromethane or N,N-4-dimethylformamide, and the reaction temperature is from 0°C to 80°C, preferably 0°C-50°C, the reaction time is 1 hour; the reaction solution is slowly added dropwise to the mixture of decitabine and N,N-4-dimethylaminopyridine. After the addition is complete, continue to react for 12 hours at a temperature of 0°C-40°C, preferably 10-30°C; Wash with distilled water, saturated sodium bicarbonate and saturated saline, collect the organic layer, dry the column with sodium persulfate, evaporate the filtrate to dryness, add Pd / C as a catalyst under the solvent of ethyl acetate, dichloromethane or isopropanol, and Catalytic hydrogenation was carried out under hydrogen conditions, the reaction time was 6 hours, suction filtration, and the filtrate was evaporated under reduced pressure to obtain the compound ( ).
Embodiment 3
[0037] Reaction of N-benzyloxycarbonyl-L-isoleucine with dicyclohexylcarbodiimide, the reaction solvent is anhydrous tetrahydrofuran, dichloromethane or N,N-4-dimethylformamide, the reaction temperature is 0℃ to 80°C, preferably 0°C-50°C, and the reaction time is 1 hour; slowly add the reaction liquid dropwise into the mixed liquid of decitabine and N,N-4-dimethylaminopyridine. After the addition is complete, continue to react for 12 hours at a temperature of 0°C-40°C, preferably 10-30°C; Wash with distilled water, saturated sodium bicarbonate and saturated saline, collect the organic layer, dry the column with sodium persulfate, evaporate the filtrate to dryness, add Pd / C as a catalyst under the solvent of ethyl acetate, dichloromethane or isopropanol, and Catalytic hydrogenation was carried out under hydrogen conditions, the reaction time was 6 hours, suction filtration, and the filtrate was evaporated under reduced pressure to obtain the compound ( ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com