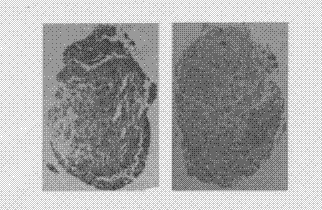
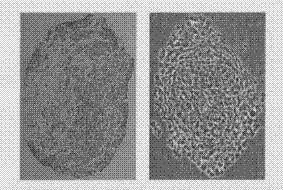
Method for differentiating induced pluripotent stem (iPS) cells into cartilage cells
A technology of pluripotent stem cells and chondrocytes, which is applied in the field of differentiation of induced pluripotent stem cells into chondrocytes, can solve the problems of high price, complicated induction system, and poor repeatability of the operating system, so as to reduce the probability of pollution, advanced and simple technology, Effect of reducing cell damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) Preparation of primary embryonic mouse fibroblasts
[0017] Under sterile conditions, take the fetuses of BABL / C mice at 13-14 days of pregnancy, remove the embryo head and viscera, cut the fetuses into paste with ophthalmic scissors, put them in 0.25% trypsin, and put them in a water bath at 37°C Digest for 15 minutes, pour the upper cell suspension into a 10ml centrifuge tube, and centrifuge at 1000r / min for 5 minutes to collect the cells. The obtained cells are made into a cell suspension with 10% fetal bovine serum DMEM medium, and 1×10 6 Inoculated into 100mm Petri dishes at a density of 37°C, 5% CO 2 Cultured in an incubator, about 2-3 days after the cells are full, the cells can be collected and frozen in liquid nitrogen. The high-sugar DEME medium is a disclosed medium composition, and commercially available products, such as Gibco or Sigma, can be used.
[0018] (2) Preparation of feeder cells
[0019] Resuscitate the embryonic mouse fibroblasts prepared...
Embodiment 2
[0034] (1) Preparation of primary embryonic mouse fibroblasts
[0035] Under sterile conditions, take the fetuses of BABL / C mice at 13-14 days of pregnancy, remove the embryo head and viscera, cut the fetuses into paste with ophthalmic scissors, put them in 0.25% trypsin, and put them in a water bath at 37°C Digest for 15 minutes, pour the upper cell suspension into a 10ml centrifuge tube, and centrifuge at 1000r / min for 5 minutes to collect the cells. The obtained cells are made into a cell suspension with 10% fetal bovine serum DMEM medium, and 1×10 6 Inoculated into 100mm Petri dishes at a density of 37°C, 5% CO 2 Cultured in an incubator, about 2-3 days after the cells are full, the cells can be collected and frozen in liquid nitrogen. The high-sugar DEME medium is a disclosed medium composition, and commercially available products, such as Gibco or Sigma, can be used.
[0036] (2) Preparation of feeder cells
[0037] Resuscitate the embryonic mouse fibroblasts prepared...
Embodiment 3
[0046] (1) Preparation of primary embryonic mouse fibroblasts
[0047] Under sterile conditions, take the fetuses of BABL / C mice at 13-14 days of pregnancy, remove the embryo head and viscera, cut the fetuses into paste with ophthalmic scissors, put them in 0.25% trypsin, and put them in a water bath at 37°C Digest for 15 minutes, pour the upper cell suspension into a 10ml centrifuge tube, and centrifuge at 1000r / min for 5 minutes to collect the cells. The obtained cells are made into a cell suspension with 10% fetal bovine serum DMEM medium, and 1×10 6 Inoculated into 100mm Petri dishes at a density of 37°C, 5% CO 2 Cultured in an incubator, about 2-3 days after the cells are full, the cells can be collected and frozen in liquid nitrogen. The high-sugar DEME medium is a disclosed medium composition, and commercially available products, such as Gibco or Sigma, can be used.
[0048] (2) Preparation of feeder cells
[0049] Resuscitate the embryonic mouse fibroblasts prepared...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com