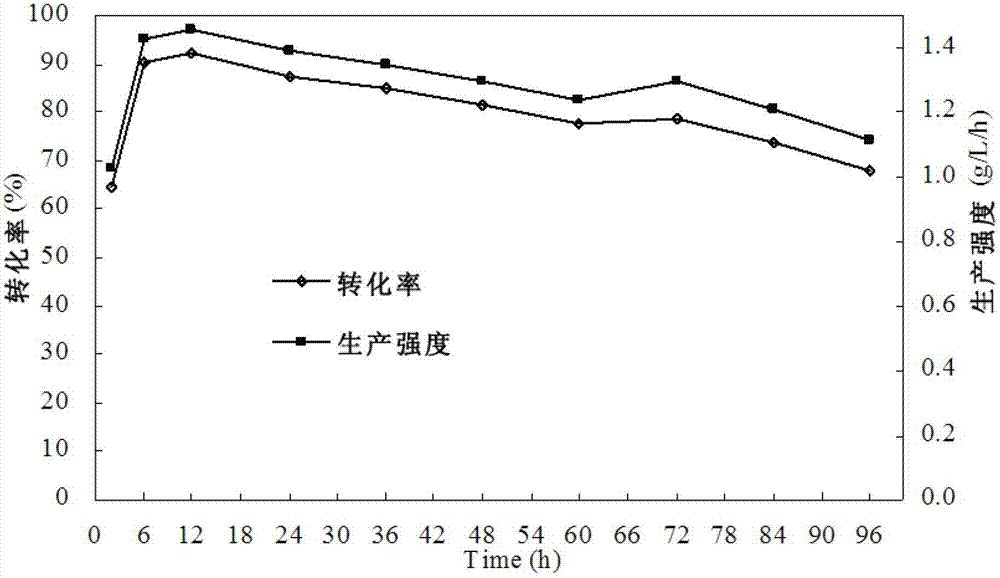
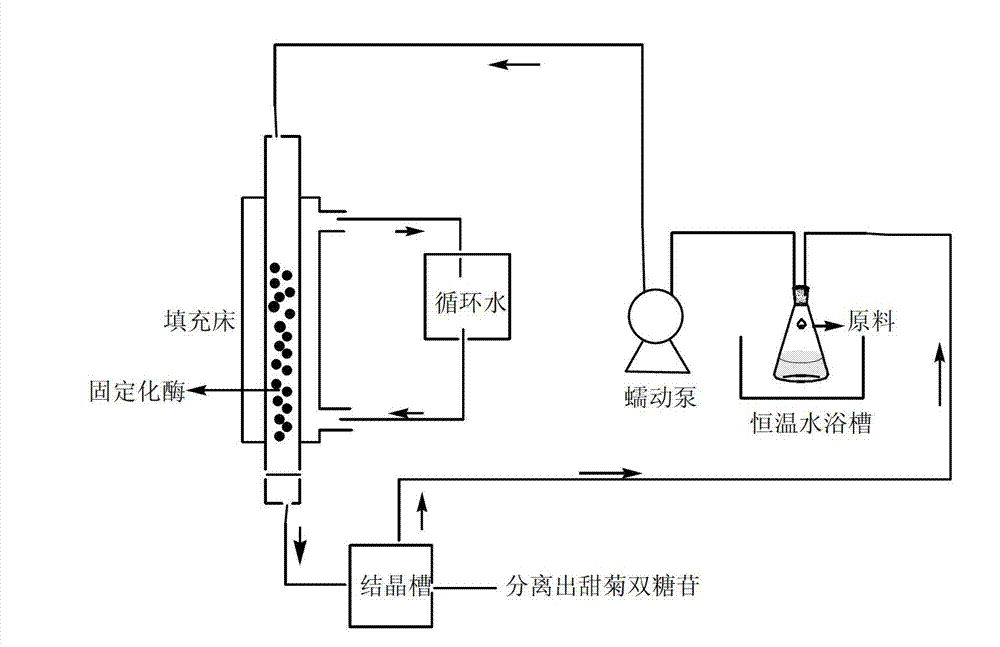
Method for preparing rebaudioside by use of stevioside
A technology of steviolbioside and stevioside, which is applied in the direction of fixing on/in organic carrier, fermentation, etc., can solve the problems of short service life and long reaction time of β-glucosidase, and achieve high economic value and high Selectivity, effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: Preparation of steviolbioside
[0081] The implementation steps of this embodiment are as follows:
[0082] Firstly, immobilized β-glucosidase is prepared.
[0083] A. Preparation of transparent gel
[0084] According to the ratio of the weight of chitosan in g to the volume of acetic acid aqueous solution in mL of 0.3:20, chitosan was dissolved in 2% by weight of acetic acid aqueous solution, and then the bubbles were removed by ultrasonic waves to obtain a transparent chitosan gel.
[0085] B. Preparation of chitosan microspheres
[0086]Add the transparent chitosan gel obtained in step A into a coagulation solution, then harden at a temperature of 4°C for 14 hours, then wash with deionized water until neutral, and store it overnight at a temperature of 4°C to obtain a chitosan microspheres.
[0087] The coagulation solution is composed of 10% by weight NaOH aqueous solution and 95% by weight ethanol aqueous solution according to the volume ratio of ...
Embodiment 2
[0095] Embodiment 2: Preparation of steviolbioside
[0096] The implementation steps of this embodiment are as follows:
[0097] Firstly, immobilized β-glucosidase is prepared.
[0098] A. Preparation of transparent gel
[0099] According to the ratio of the weight of chitosan microspheres in g to the volume of glutaraldehyde aqueous solution in mL 0.2:24, chitosan was dissolved in 5% by weight acetic acid aqueous solution, and then the bubbles were removed by ultrasonic method to obtain a transparent shell Glycan Gel.
[0100] B. Preparation of chitosan microspheres
[0101] Add the transparent chitosan gel obtained in step A into a coagulation solution, then harden at a temperature of 2°C for 16 hours, then wash with deionized water until neutral, and store it overnight at a temperature of 3°C to obtain a chitosan microspheres.
[0102] The coagulation solution is composed of 8% by weight NaOH aqueous solution and 98% by weight ethanol aqueous solution according to the ...
Embodiment 3
[0107] Embodiment 3: Preparation of steviolbioside
[0108] The implementation steps of this embodiment are as follows:
[0109] Firstly, immobilized β-glucosidase is prepared.
[0110] A. Preparation of transparent gel
[0111] According to the ratio of the weight of chitosan microspheres in g to the volume of glutaraldehyde aqueous solution in mL of 0.5:16, chitosan was dissolved in 1% by weight acetic acid aqueous solution, and then the bubbles were removed by ultrasonic method to obtain a transparent shell. Glycan Gel.
[0112] B. Preparation of chitosan microspheres
[0113] Add the transparent chitosan gel obtained in step A into a coagulation solution, then harden at a temperature of 6°C for 10 hours, then wash with deionized water until neutral, and store it overnight at a temperature of 3°C to obtain a chitosan microspheres.
[0114] The coagulation solution is composed of 12% by weight NaOH aqueous solution and 92% by weight ethanol aqueous solution according to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com