Octreotide sustained-release microspheres with high encapsulation rate and preparation method thereof
A technology of slow-release microspheres and octreotide, which is applied in the direction of pharmaceutical formulations, peptide/protein ingredients, and medical preparations of non-active ingredients, etc., can solve problems such as unsatisfactory results, and achieve round shape, smooth surface, and excellent preparation technology. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
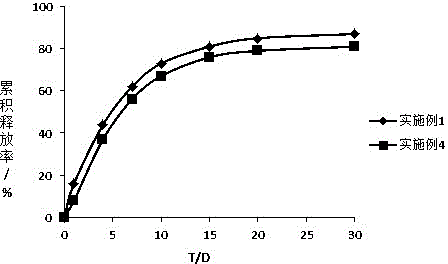
Embodiment 1
[0025] Prescription process composition:
[0026] Prescription process content or ratio Octreotide: PLGA(w / w) (PLGA Mw=38000, 50:50poly, i.v.=0.61dL / g) 1:5.6 Trehalose: Octreotide (w / w) 1:2 Continuous phase pH 7.92 Polyvinyl alcohol (mg / ml) 10 Polysorbate 80(mg / ml) 0.2
[0027] The preparation method of octreotide sustained-release microspheres is as follows:
[0028] Dissolve 196mg PLGA in dichloromethane as the oil phase; dissolve 35mg octreotide and stabilizer trehalose 17.5mg in water as the dispersed phase; dissolve 400mg polyvinyl alcohol and 8mg polysorbate 80 in 40ml phosphate buffer as the solvent for the continuous phase; mix and oscillate the dispersed phase with the oil phase to form colostrum, add the colostrum to the continuous phase under stirring to form double emulsion; then quickly transfer the double emulsion to phosphate buffer, stir magnetically until the dichloromethane volatilizes Completely, filter and coll...
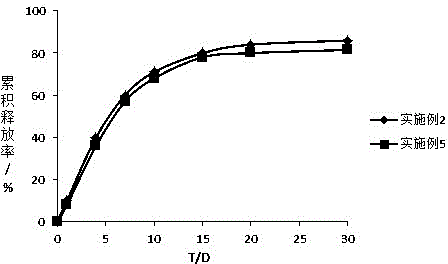
Embodiment 2
[0040] Prescription process composition:
[0041] Prescription process content or ratio Octreotide: PLGA(w / w) (PLGA Mw=38000, 50:50poly, i.v.=0.61dL / g) 1:8 Trehalose: Octreotide (w / w) 1:3 Continuous phase pH 7.8 Polyvinyl alcohol (mg / ml) 20 Polysorbate 80(mg / ml) 0.4
[0042] The preparation method of octreotide sustained-release microspheres is as follows:
[0043]Dissolve 196mg of PLGA in dichloromethane as the oil phase; dissolve 24.5mg of octreotide in water as the dispersed phase, which contains 2.45mg of stabilizer trehalose; dissolve 800mg of polyvinyl alcohol and 16mg of polysorbate in 40ml of phosphate buffer as the solvent 80 is the continuous phase; the dispersed phase and the oil phase are mixed and shaken to form colostrum, then added to the continuous phase under stirring to form double emulsion, and then quickly transferred to a certain volume of phosphate buffer, magnetically stirred until the dichloromethane volatil...
Embodiment 3
[0046] Prescription process composition:
[0047] Prescription process content or ratio Octreotide: PLGA(w / w) (PLGA Mw=79300, 75:25poly, i.v.=0.55dL / g) 1:10 Trehalose: Octreotide (w / w) 1:5 Continuous phase pH 7.4 Polyvinyl alcohol (mg / ml) 8 Polysorbate 80(mg / ml) 0.16
[0048] The preparation method of octreotide sustained-release microspheres is as follows:
[0049] Dissolve 196mg of PLGA in dichloromethane as the oil phase; dissolve 19.6mg of octreotide in water as the dispersed phase, which contains 3.92mg of stabilizer trehalose; dissolve 320mg of polyvinyl alcohol and 6.4mg of polysorbate in 40ml of phosphate buffer as the solvent Ester 80 is the continuous phase; the dispersed phase and the oil phase are mixed and shaken to form colostrum, then added to the continuous phase under stirring to form double emulsion, and then quickly transferred to a certain volume of phosphate buffer, magnetically stirred until dichloromethane A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com