Pharmaceutical compositions of co-crystals of tramadol and coxibs
A technology of composition and co-crystal, which is applied in the direction of drug combination, active ingredients of hydroxyl compounds, antipyretic drugs, etc., to achieve the effects of enhancing drug release rate, increasing bioavailability, and enhancing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
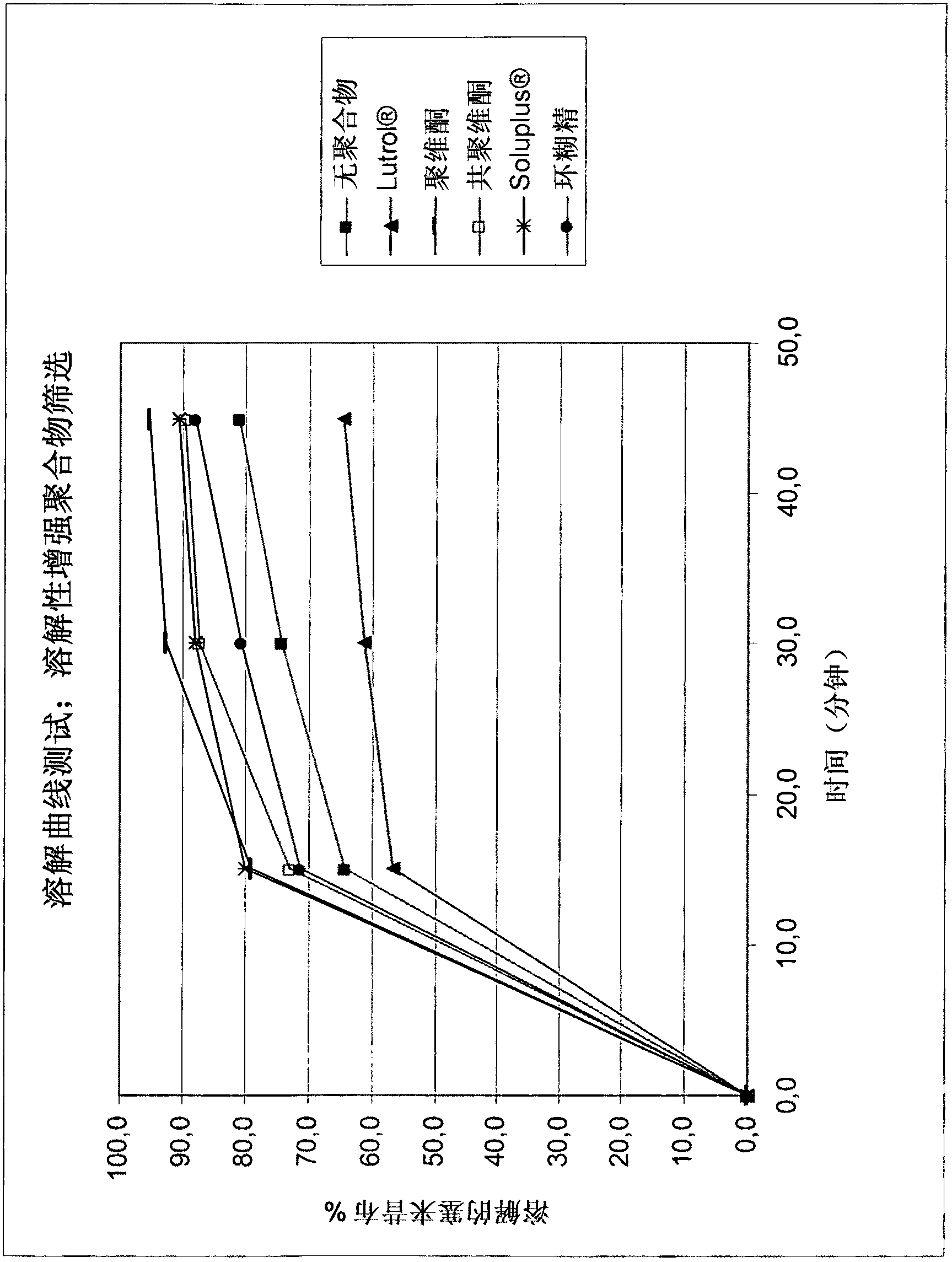
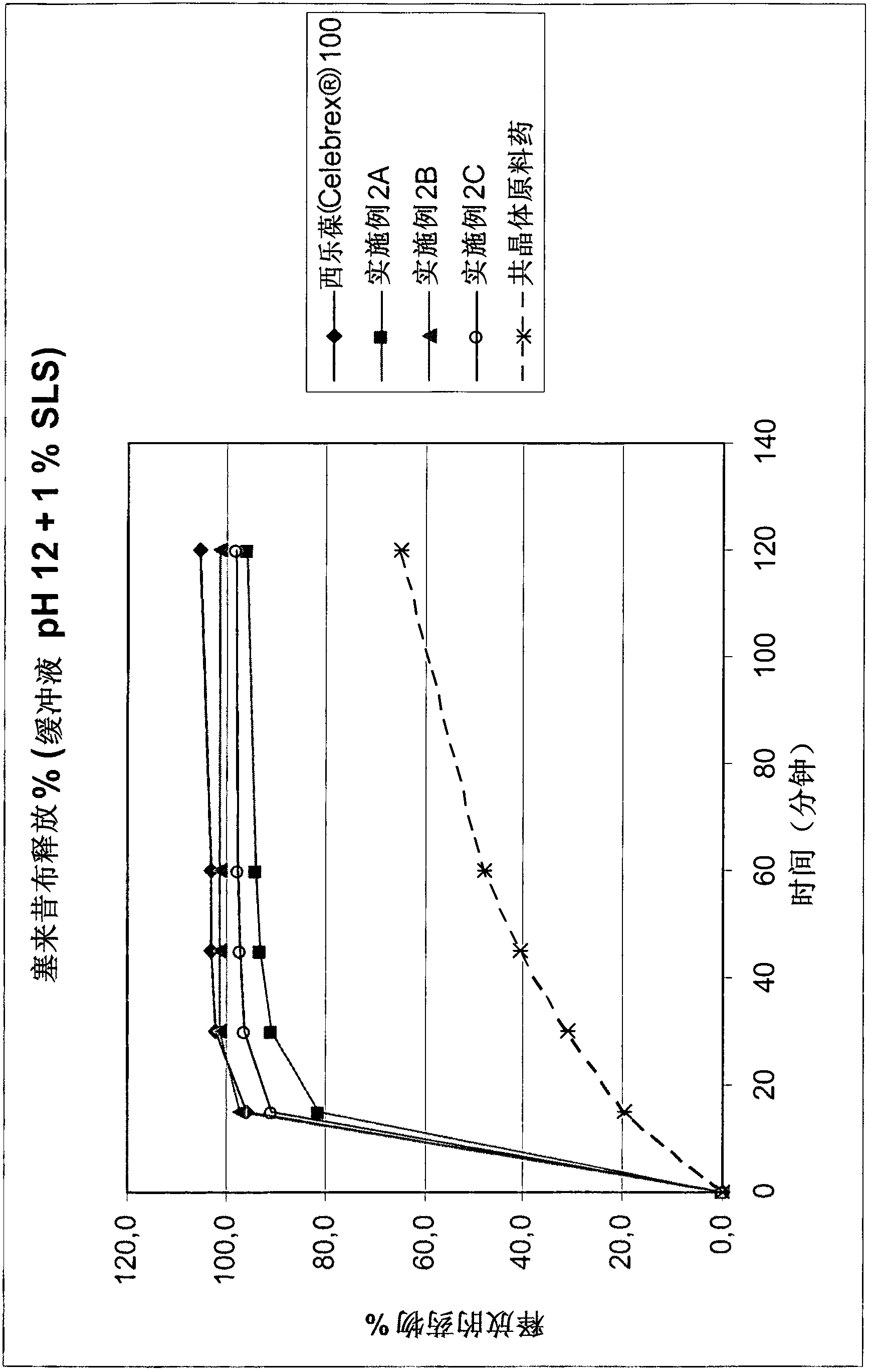
[0041] In a preferred embodiment, the fluid (such as containing 2% SLS (sodium lauryl sulfate; S odium L auryl S ulphate) in 0.1N HCl solution or pH 12 buffer solution containing 1% SLS) (racemic)-tramadol HCl-celecoxib (1:1 ) percentage of co-crystals will be greater than 85% within the first 30 minutes and / or will be greater than 90% within the first 45 minutes (eg in the USP paddle test).
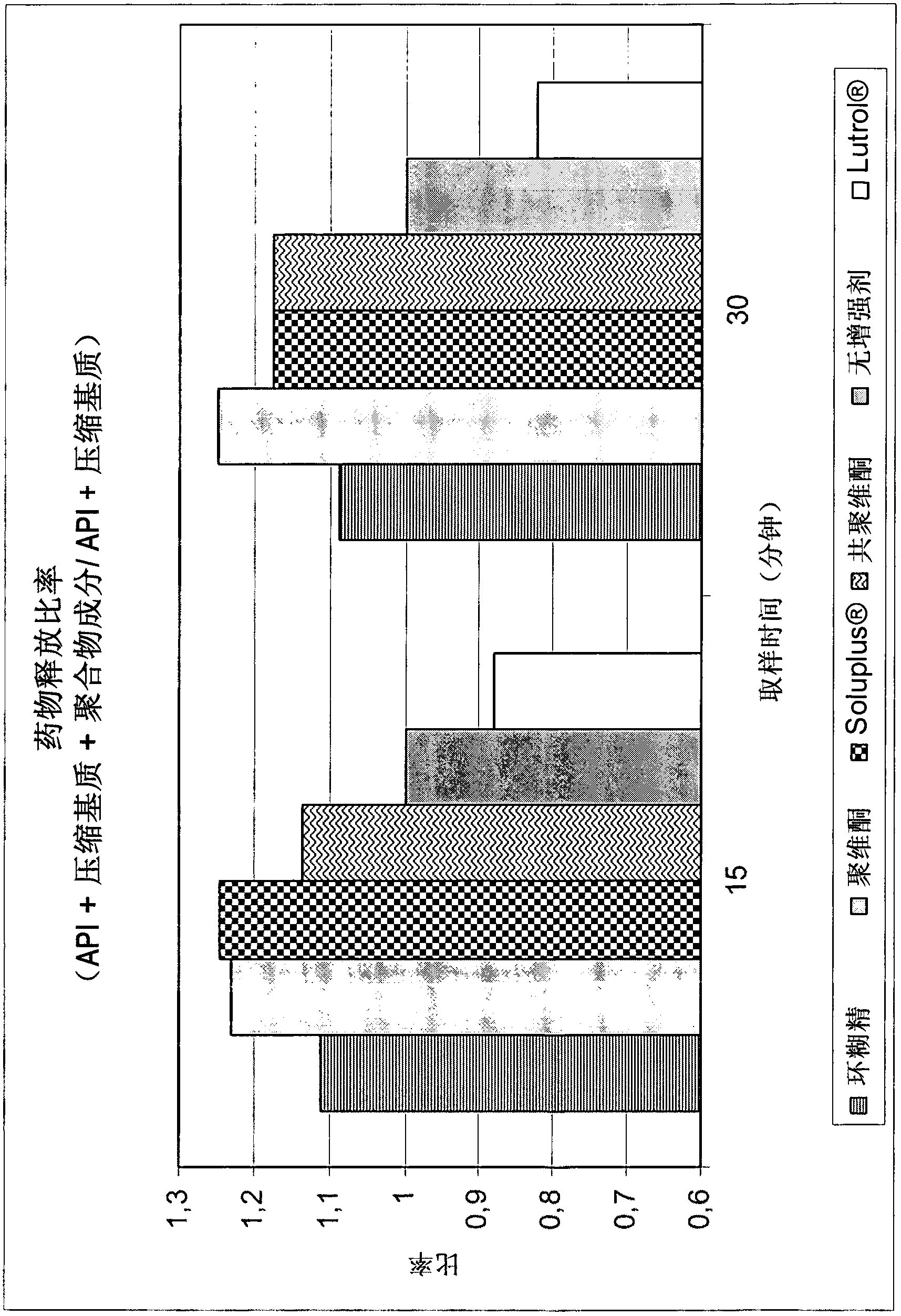
[0042] In a preferred embodiment, "solubility enhancing polymer" is selected from caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer or other hydrophilic polymers such as copovidone (copolymer of 1-vinyl-2-pyrrolidone and vinyl acetate; VA64), povidone, poloxamer (nonionic polyoxyethylene-polyoxypropylene copolymer, e.g. ), cyclodextrin Polyethylene glycol and its derivatives (PEG) and glyceryl behenate Or selected from polyethylene caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer or other hydrophilic polymers such as copovidone (copolymer of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com