Preparation method of ZnO nanocrystals
A nanocrystal, 2·6H2O technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of complex operation, harsh conditions, expensive equipment, etc., and achieve simple operation process, reaction Low temperature, easy to scale effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
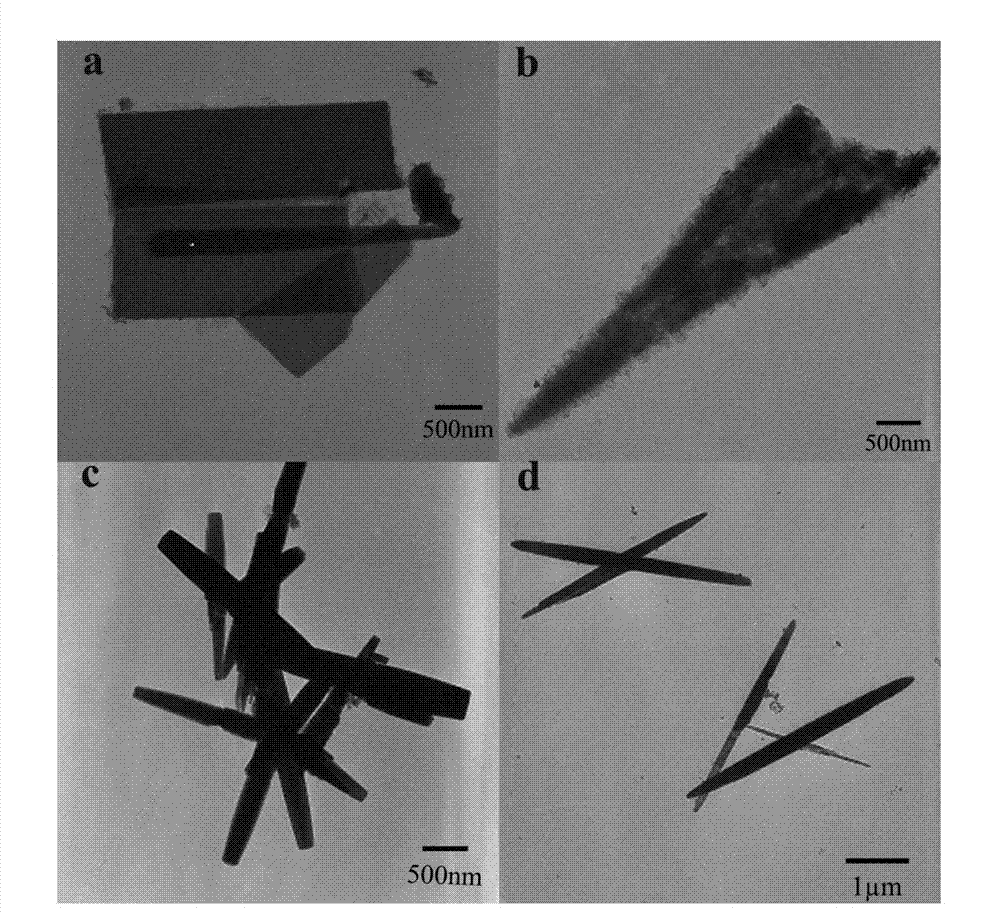
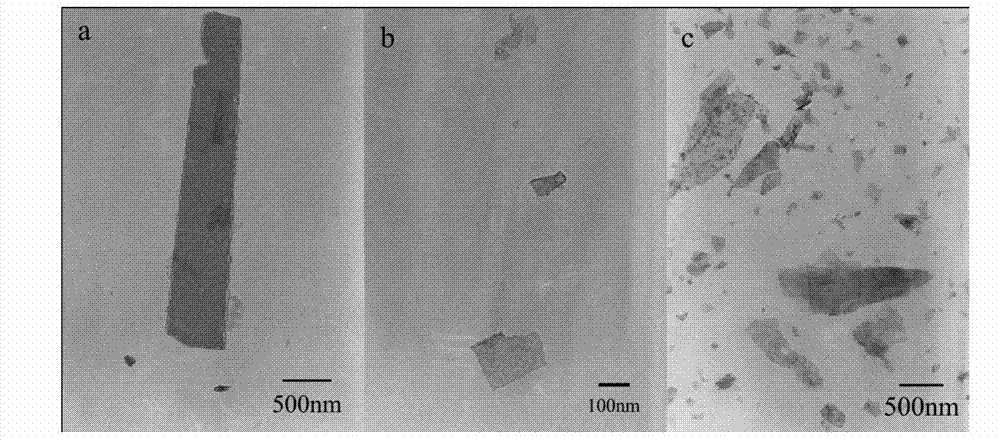
[0027] Example 1, adjusting the concentration of precursors to prepare ZnO nanocrystals
[0028] Weigh 1.190g (4mmol) of Zn(NO 3 ) 2 ·6H 2 O and 0.561g (4mmol) of HMT (hexamethylenetetramine), add in the small beaker, measure 20mL deionized water and add in the beaker, then put the magnet into the small beaker, stir for 0.5h to make the solution well mixed. Pipette 10mL, 2.5mL, 1mL and 0.15mL of the precursor solution from the above beakers with a pipette gun, and add them to the four beakers respectively. Then add solvent ethanol to the four beakers in turn, so that the solvents all contain 10 mL of water and 20 mL of ethanol. Put the four beakers into a digitally controlled ultrasonic cleaner, put them in an ultrasonic bath for 0.5h, and then transfer them to four polytetrafluoroethylene reaction kettles in turn. Set the oven temperature to 100°C, put the reactor into it, and control the reaction time to 24h. After the reaction, the reactor was taken out and cooled nat...
Embodiment 2
[0030] Embodiment 2, control reaction time prepares ZnO nanocrystal
[0031] Weigh 0.476g (1.6mmol) of Zn(NO 3 ) 2 ·6H 2 Add O and 0.224g (1.6mmol) of HMT (hexamethylenetetramine) into a small beaker, measure 80mL of deionized water into it, then put the magnet into the small beaker, and stir for 0.5h to make the solution evenly mixed. Use a pipette gun to pipette 10 mL of the precursor solution from the above beakers and add them to the six beakers respectively. Then add solvents to these six beakers in turn, so that the solvents all contain 10 mL of water and 20 mL of ethanol. Put the six beakers into a digitally controlled ultrasonic cleaner, put them in an ultrasonic bath for 0.5h, and then transfer them to six polytetrafluoroethylene reaction kettles in turn. Set the oven temperature to 100°C, put all the reactors into it, and adjust the reaction time to 1~16h. According to the reaction time, take out the corresponding numbered reactors one by one, and let them cool ...
Embodiment 3
[0033] Embodiment 3, select different solvents to prepare ZnO nanocrystals
[0034] Weigh 2.380g (8mmol) of Zn(NO 3 ) 2 ·6H 2 Add O and 1.121g (8mmol) of HMT (hexamethylenetetramine) into a small beaker, measure 40mL of deionized water into it, then put the magnet into the beaker, and stir for 0.5h to make the solution evenly mixed. Use a pipette gun to pipette 10 mL of the precursor solution from the above beakers and add them to the three beakers respectively. Then add solvents to the three beakers in turn, so that the solvents all contain 10 mL of water and 20 mL of ethanol. Put the three beakers into a digitally controlled ultrasonic cleaner, put them in an ultrasonic bath for 0.5h, and then transfer them to three polytetrafluoroethylene reaction kettles in turn. Set the oven temperature to 150°C, put all the reactors into it, and control the reaction time to 24h. After the reaction, the reactor was taken out and cooled naturally at room temperature. The reaction sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com