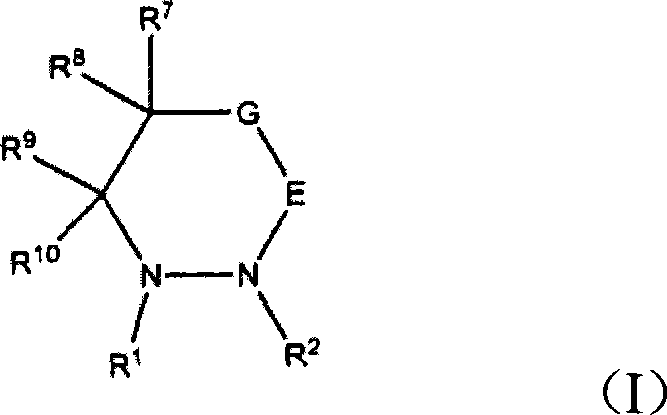
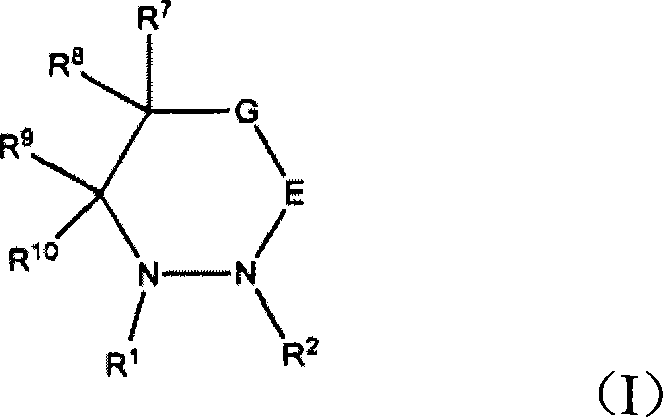
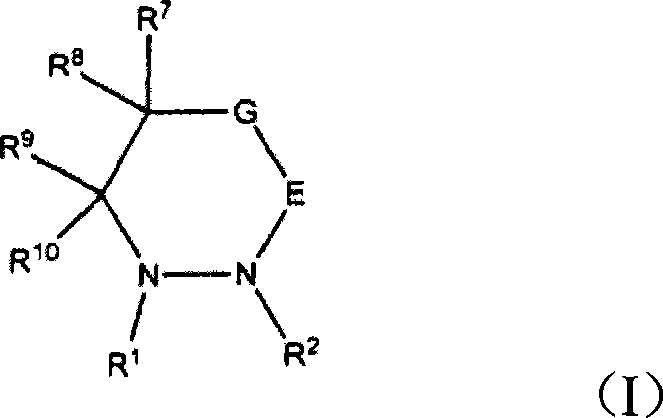
Electroplating solution and electroplating method
A copper electroplating solution and electrolyte technology, applied in the fields of metal electroplating and copper electroplating, can solve the problem of not being able to provide a flat copper plating layer, and achieve the effect of reducing nodules and good dispersing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] In a 100 mL round-bottomed three-necked flask equipped with a condenser and a thermometer, add 100 mmol of pyrazole and 20 mL of deionized water, and then add 63 mmol of 1,4-butanediol diglycidyl ether. The resulting mixture was heated using an oil bath set to 110° C. for about 5 hours and stirred at room temperature for an additional 8 hours. The amber, less viscous reaction product was transferred to a 200 mL volumetric flask, rinsed and adjusted to the 200 mL mark with deionized water. The reaction product (reaction product 1) was used without further purification. reaction product 1 1 HNMR (500MHz, CH 3OH-d6) analysis results showed the following peaks confirming its structure: δppm: 7.65-7.62 (m, 1H, H arom .); 7.49-7.48 (m, 1H, H arom .); 6.29-6.27 (m, 1H, H arom .); 4.32-3.30(m, 8.82H(14Hx0.63mole), 4xCH 2 -O, 2xCH-OH, 2xCH 2 -N); 1.69-1.63(m, 2.52H(4Hx0.63mole), 2xCH 2 ).
Embodiment 2
[0084] 1,4-Butanediol diglycidyl ether (25.2 mmol) and 40 mmol of 4,5,6,7-tetrahydroindazole were added to a round bottom reaction flask at room temperature. 12 mL of deionized water was then added to the flask. The initially formed white suspension eventually transformed into a phase-separated mixture as the reaction temperature increased. The reaction mixture was heated using an oil bath set to 95°C for about 2 hours. After that, 2 mL of concentrated sulfuric acid (or 4 mL of 50% sulfuric acid) was added to the reaction flask, and the solution became transparent light yellow. The mixture was heated for an additional 3 hours and stirred at room temperature for an additional 8 hours. The resulting light amber reaction product was transferred to a volumetric flask, rinsed and diluted with 0.5-1% sulfuric acid. The reaction product (reaction product 2) solution was used without further purification.
Embodiment 3
[0086] The reaction products in Table 1 were prepared by the general procedure of Example 1 or 2. Detect the ultraviolet absorption spectrum of this reaction product in water and record the λ of absorbance in table 1 max (nm).
[0087] Table 1
[0088]
[0089]
[0090]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com