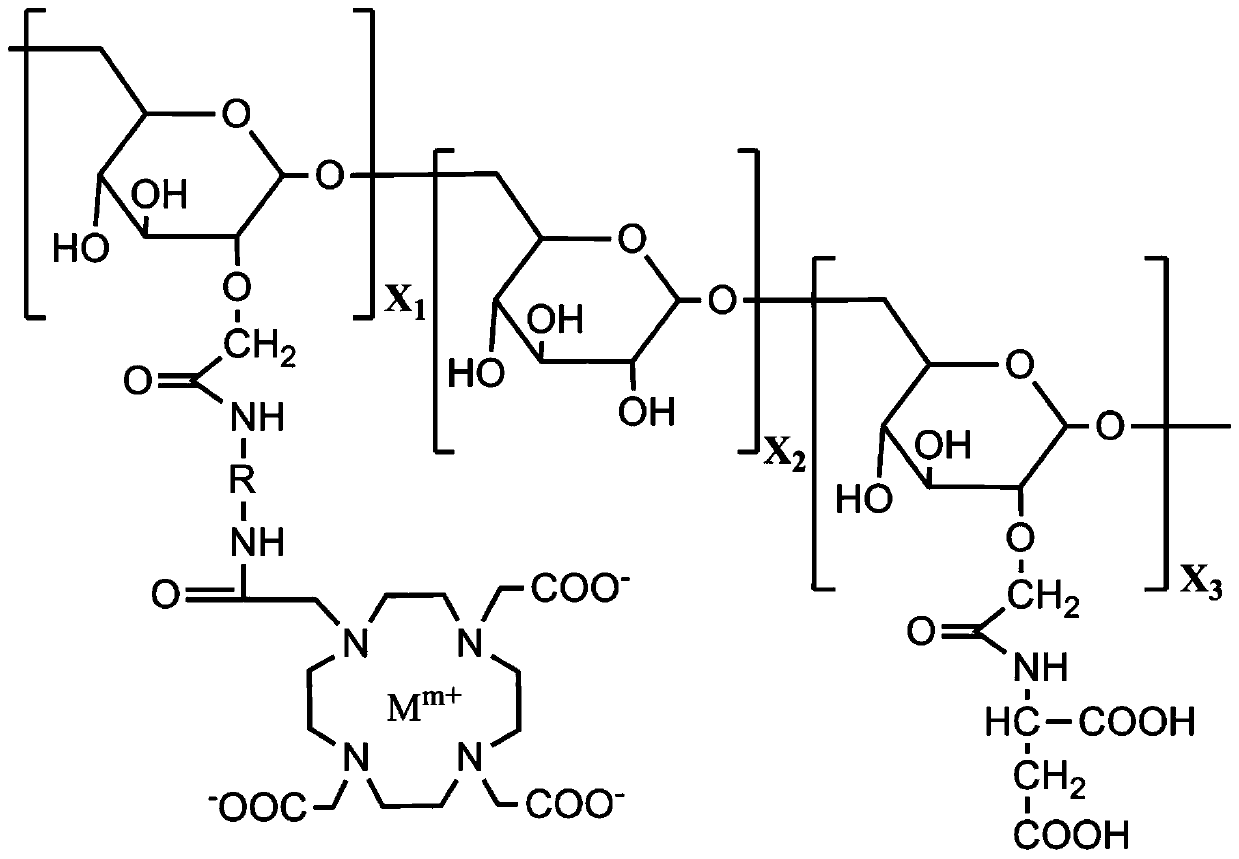
Paramagnetism metal complex magnetic resonance imaging contrast agent containing asparaginic acid-glucan and preparation method of contrast agent
A technology of aspartic acid and paramagnetic metals, which is applied in the preparation of X-ray contrast agents, echo/ultrasonic imaging agents, preparations for in vivo tests, etc., can solve the problems of low relaxation efficiency of contrast agents, and achieve improved water solubility. properties, high relaxation efficiency, and improved safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of the paramagnetic metal complex magnetic resonance imaging contrast agent containing aspartic acid-dextran of the present invention, the preparation method specifically comprises the following steps:
[0038] (1) Dissolve dextran in sodium hydroxide solution at a temperature of 0~4°C, then add monochloroacetic acid, the mass ratio of monochloroacetic acid to dextran is 1.5:1, and stir for 1~3 hours, Obtain carboxymethylated dextran;
[0039] (2) Dissolve the carboxymethylated dextran synthesized in (1) in water at room temperature, adjust the pH of the solution to 3.0~4.5 with hydrochloric acid, add 2-ethoxy-1-ethoxycarbonyl- 1,2-dihydroquinoline (EEDQ), the mass ratio of EEDQ and carboxymethylated dextran is 1.1:2, add ethylenediamine, the mass of ethylenediamine and carboxymethylated dextran The ratio is 5:1, and after fully reacting, aminated dextran is obtained;
[0040] (3) Dissolve 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid...
Embodiment 1
[0045] Aspartic acid-dextran-containing 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid macromolecular gadolinium paramagnetic metal complex contrast agent for magnetic resonance imaging preparation
[0046] (1) Dissolve dextran in 6mol / L sodium hydroxide solution at 0 °C, stir well, add isopropanol drop by drop, stir for 30 minutes, add monochloroacetic acid, monochloroacetic acid and dextran The mass ratio was 1.5:1, the temperature was raised to 60°C and stirred for 1 hour, precipitated with methanol, filtered, dialyzed, and freeze-dried to obtain carboxymethylated dextran;
[0047] (2) Dissolve the carboxymethylated dextran synthesized in (1) in water at room temperature, adjust the pH of the solution to 3.0 with 1mol / L hydrochloric acid, add EEDQ solution dropwise, EEDQ and carboxymethylated The mass ratio of dextran is 1.1:2, after stirring evenly, add ethylenediamine, the mass ratio of ethylenediamine and carboxymethylated dextran is 5:1, react for 4 hours, an...
Embodiment 2
[0053] Aspartic acid-dextran-containing 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid macromolecular gadolinium paramagnetic metal complex contrast agent for magnetic resonance imaging preparation
[0054] (1) Dissolve dextran in 6mol / L sodium hydroxide solution at 4°C, stir well, add isopropanol drop by drop, stir for 30 minutes, add monochloroacetic acid, monochloroacetic acid and dextran The mass ratio is 1.5:1, raise the temperature to stir for 2 hours, precipitate with methanol, filter, dialyze, and freeze-dry to obtain carboxymethylated dextran;
[0055] (2) Dissolve the carboxymethylated dextran synthesized in (1) in water at room temperature, adjust the pH of the solution to 4.5 with 1mol / L hydrochloric acid, add EEDQ solution dropwise, EEDQ and carboxymethylated The mass ratio of dextran is 1.1:2, after stirring evenly, add ethylenediamine, the mass ratio of ethylenediamine and carboxymethylated dextran is 5:1, react for 4 hours, and the product is precipi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com