High-barrier multi-layer coextrusion composite infusion film
A multi-layer co-extrusion, infusion film technology, applied in the direction of layered products, transportation and packaging, synthetic resin layered products, etc., can solve the problems of not being widely used, wasting energy, and high surface tension of the adhesive layer.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention will be further described below in conjunction with specific embodiments. Unless otherwise specified, the raw materials used in the present invention are all commercially available, and all production and preparation processes are carried out in a class 10,000 clean area (combined with polymer materials).
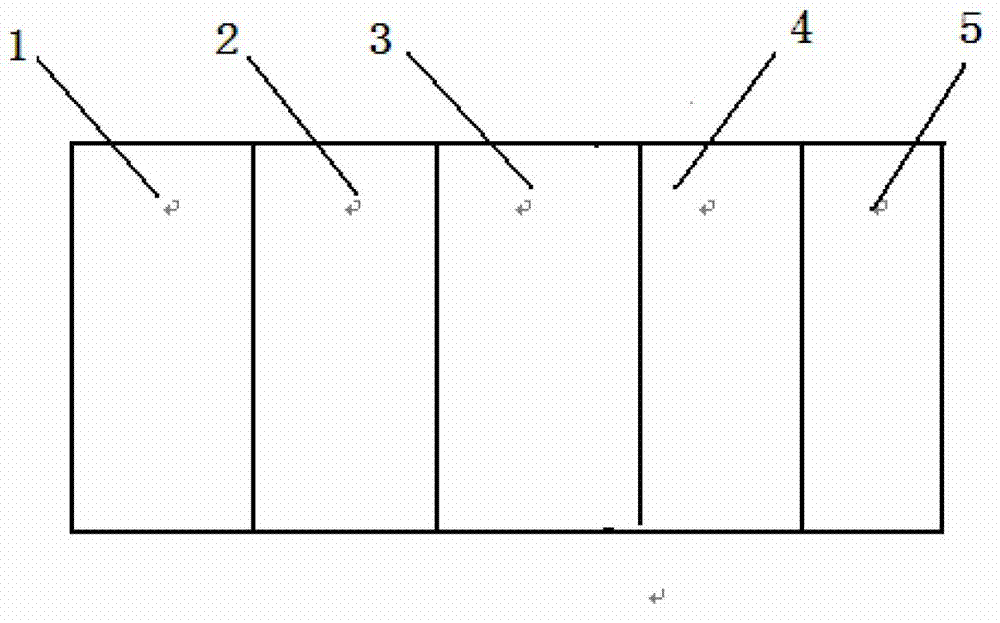
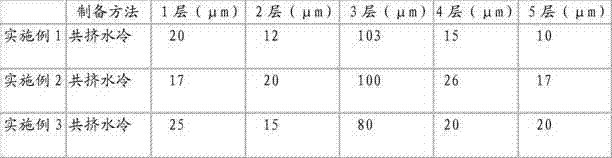
[0031] A down-blown water-cooled co-extrusion process is used to prepare multiple rolls of high-temperature sterilization-resistant medical device blister packaging films, and the thickness of the film is 160 μm, 180 μm, and 160 μm. Its specific implementation is shown in Table 1.
[0032] Table 1
[0033]
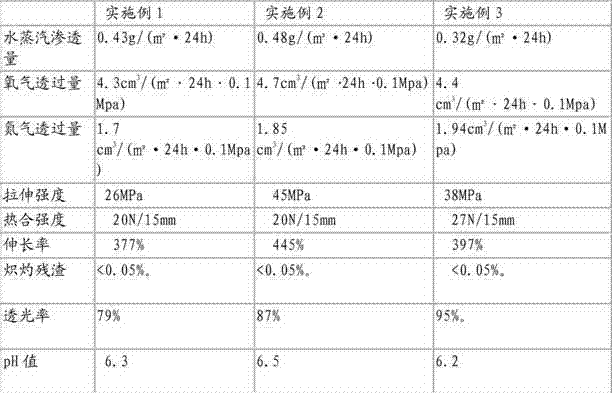
[0034] After corona treatment, each roll of film material prepared above was heat-sealed with medical transparent paper produced by Zhejiang Hengda Paper Co., Ltd., and its performance was tested. The details are shown in Table 2:
[0035] Table 2
[0036]
[0037] Refer to GBT19633-2005 for the packaging of terminally sterilized ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com