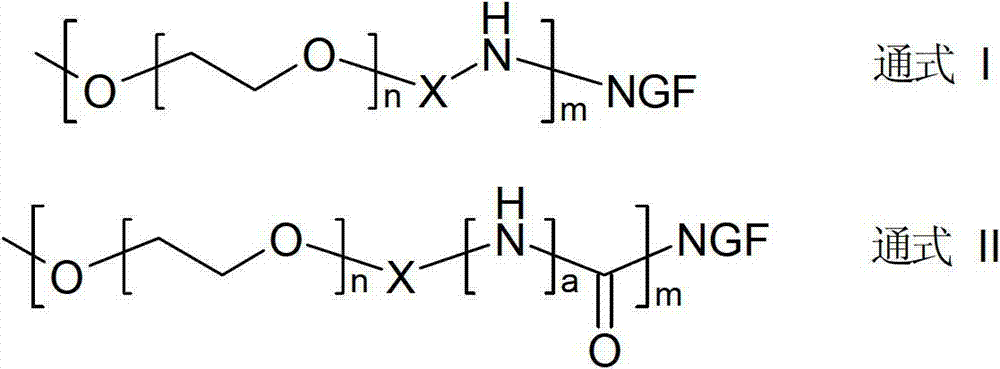
Method for preparing polyethylene glycol (PEG) and nerve growth factor (NGF) conjugate
A nerve growth factor and polyethylene glycol technology, applied in the field of biochemistry, can solve the problems of short plasma half-life and pain, and achieve the effect of reducing the frequency of administration and improving the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of Nerve Growth Factor Modified by mPEG-Succinimidyl Valerate [mPEG-SVA]
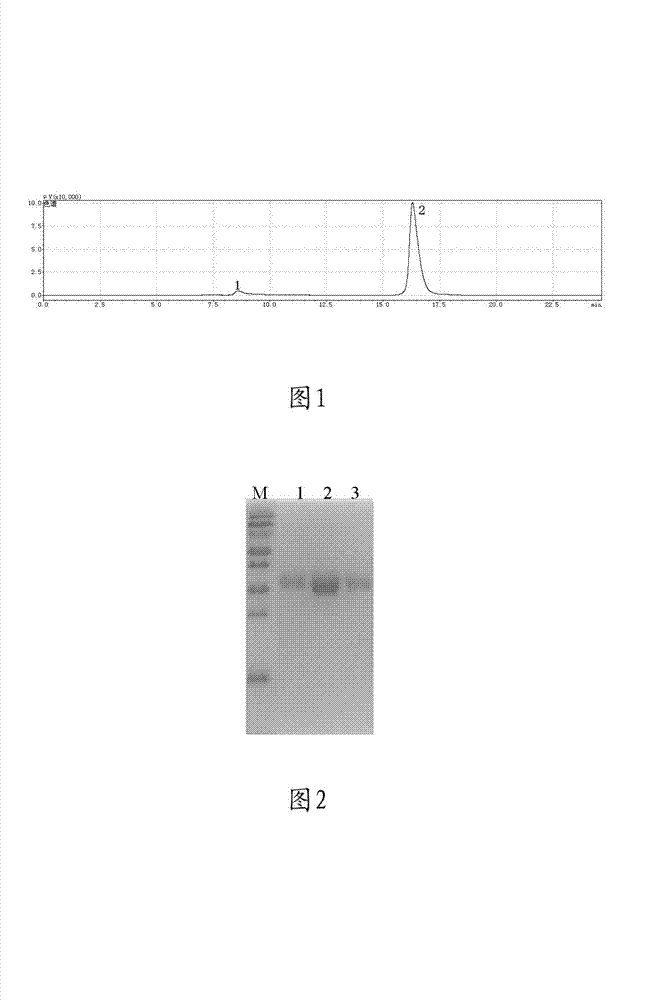
[0049] Nerve growth factor PBS solution with a concentration of 0.68 mg / mL (the PBS solution of the company’s marketed product Enjingfu) and mPEG-SVA (manufactured by Laysan Bio) dissolved in a PBS solution with a pH of 7.4, with a material ratio of 1 :10 (NGF:PEG) and stirred at room temperature for 24 hours. The PEG-modified protein is run on a gel chromatography column (such as KW802.5) was purified by chromatography using 0.05M PBS buffer solution. The results are shown in Figure 1. It can be seen from the figure that the chromatographic peak 1 is the nerve growth factor modified by mPEG-SVA, and the chromatographic peak 2 is Excess mPEG-SVA during the modification reaction. PEG-modified proteins were identified by SDS-PAGE, the results are shown in figure 2 , where lanes 1-3 represent purified PEG-modified mouse nerve growth factor, and lane M is a molecular marker...
Embodiment 2
[0050] Example 2: Preparation of Nerve Growth Factor Modified by mPEG-aldyhyde [mPEG-AD]
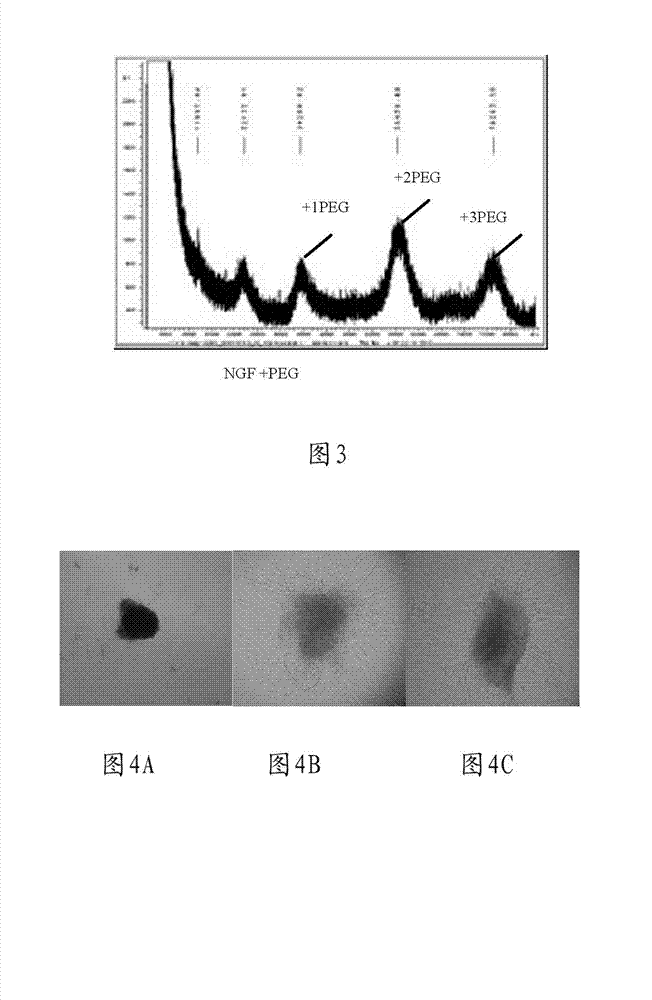
[0051] Nerve growth factor PBS solution with a concentration of 0.68 mg / mL (the PBS solution of the company’s marketed product Enjingfu) and mPEG-AD (manufactured by Laysan Bio) dissolved in a PBS solution with a pH of 6, with a material ratio of 1: 10 (NGF:PEG) ratio mixed, add 10 times the amount of NGF NaBCNH 3 , stirred at room temperature for 24 hours. The PEG-modified protein is run on a gel chromatography column (such as KW802.5) was purified by chromatography using 0.05M PBS buffer solution. PEG-modified proteins were identified by SDS-PAGE. Eluted from the column corresponding to bound a PEG (PEG 1 -NGF) or two PEGs (PEG 2 -NGF) or three PEGs (PEG 3 -NGF) NGF protein. These proteins were pooled, concentrated, and the protein concentration (7 ng / mL) was determined by colorimetric analysis. PEG-NGF was stored in 0.05M PBS (pH=7.0) buffer at -20°C.
Embodiment 3
[0052] Example 3: Preparation of Nerve Growth Factor Modified by mPEG-hydrazine [mPEG-HZ]
[0053] Method 1: Nerve growth factor solution with a concentration of 0.68 mg / mL (the PBS solution of the company’s marketed product Enjingfu) and mPEG-HZ (produced by Laysan Bio) dissolved in a PBS solution with a pH of 6 The ratio of 1:10 (NGF:PEG) was mixed, and EDAC was added with 10 times the amount of NGF, and stirred at room temperature for 24 hours. The PEG-modified protein is run on a gel chromatography column (such as KW802.5) was purified by chromatography using 0.05M PBS buffer solution. PEG-modified proteins were identified by SDS-PAGE. Eluted from the column corresponding to bound a PEG (PEG 1 -NGF) or two PEGs (PEG 2 -NGF) or three PEGs (PEG 3 -NGF) NGF protein. The proteins were pooled, concentrated and the protein concentration was determined by colorimetric analysis. PEG-NGF was stored in 0.05M PBS (pH=7.0) buffer at -20°C.
[0054] Method 2: Nerve growth fact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com