Preparation method of 2-methyl-1,2-propane diamine
A technology of propylene diamine and methyl, applied in the field of organic synthesis, can solve the problems of difficult industrialization yield, unfavorable industrialization, unstable raw materials, etc., and achieves the effects of high reaction yield, easy industrialization, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
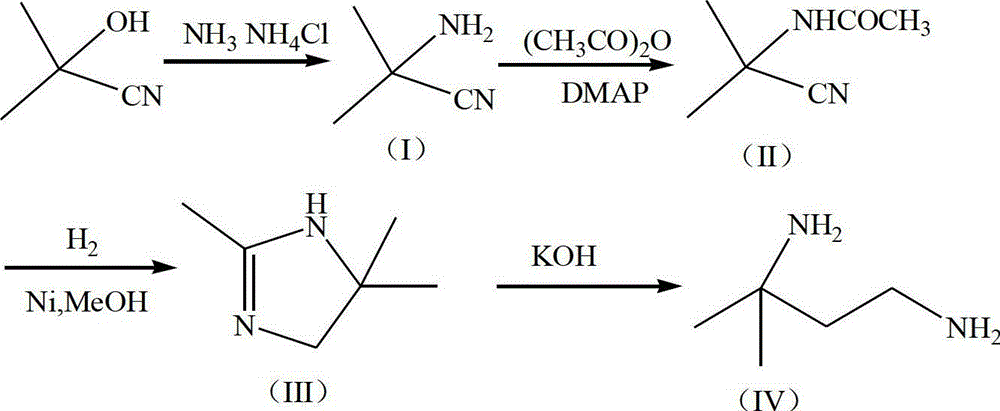
[0023] 1) Preparation of α-aminoisobutyronitrile (I)
[0024] Add 68g of ammonia water and 32.1g (0.6mol) of ammonium chloride into the reactor, stir to dissolve, add 59.5g (3.5mol) of liquid ammonia into the reactor at 0-5°C, and drop 85g (1mol) of acetone cyanohydrin at the same time, 28 React at ~30°C for 3 hours, GC detects that the raw materials have reacted completely, and the reaction is completed, add tetrahydrofuran to separate the organic layer, extract the water layer with tetrahydrofuran, combine the organic layers, dry over anhydrous sodium sulfate, filter, and collect the 60°C / 267kPa fraction by vacuum distillation of the filtrate , to obtain α-aminoisobutyronitrile (I) (colorless liquid 76.7g, yield 86.9%, content 99.9% (determined by GC area normalization method));
[0025] 2) Preparation of α-acetylaminoisobutyronitrile (II)
[0026] Add 76.5g (0.75mol) of acetic anhydride to the reactor, cool down to 0-5°C, add 25.2g (0.3mol) of α-aminoisobutyronitrile (I) d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com