Preparation method of 1-methylbenzotriazole
A technology for methylbenzotriazole and benzotriazole, which is applied in the field of preparation of 1-methylbenzotriazole, can solve the problems of less separation amount, high separation cost, complicated operation process and the like, and achieves the The effect of low cost, high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
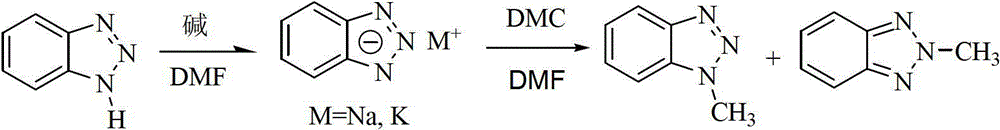
[0017] Example 1: Add 119g (1mol) benzotriazole and 275g N,N-dimethylformamide into a 1000mL three-neck round bottom flask, stir at room temperature, and add 44.0 g (1.1mol) sodium hydroxide, stirred at room temperature until the benzotriazole was converted into benzotriazole salt (followed by thin-layer chromatography). Raise the temperature to 90°C, add 144g (1.6mol) dimethyl carbonate dropwise under stirring, and react at a constant temperature until the benzotriazole salt completely participates in the reaction (tracking by thin-layer chromatography). Cool at room temperature, change the reflux device to a vacuum distillation device, collect fractions at 58-68°C (50KPa), recover 256g of N,N-methylformamide, and the recovery rate is 93%. Add 275g of water to dissolve the salt formed in the reaction process to the underpressure distillation residue, let it stand for layers, separate the organic phase, dry it with anhydrous sodium sulfate, filter, and in the filtrate, 1-methy...
Embodiment 2
[0018] Example 2: Add 119g (1mol) benzotriazole and 300g N,N-dimethylformamide into a 1000mL three-neck round bottom flask, stir at room temperature until benzotriazole is completely dissolved, then add in batches 112.0 g (1 mol) of potassium tert-butoxide was reacted until benzotriazole was converted into benzotriazole salt (followed by TLC). Raise the temperature to 100°C, add 117.0 g (1.3 mol) of dimethyl carbonate dropwise under stirring, and react at a constant temperature until the benzotriazole salt completely participates in the reaction (tracked by thin-layer chromatography). Cool at room temperature, change the reflux device to a vacuum distillation device, collect fractions at 58-68°C (50KPa), recover 273g of N,N-dimethylformamide, and the recovery rate is 91%. Add 295g of water to dissolve the salt formed in the reaction process to the underpressure distillation residue, let it stand for layers, separate the organic phase, dry it with anhydrous sodium sulfate, filt...
Embodiment 3
[0019] Example 3: Add 119g (1mol) benzotriazole and 300g N,N-dimethylformamide into a 1000mL three-neck round bottom flask, stir at room temperature until benzotriazole is completely dissolved, then add in batches 123.2 g (1.1 mol) of potassium tert-butoxide was stirred until the benzotriazole was converted to the benzotriazole salt (followed by TLC). Raise the temperature to 95°C, add 157.5 g (1.75 mol) dimethyl carbonate dropwise under stirring, and react at a constant temperature until the benzotriazole salt completely participates in the reaction (tracked by thin-layer chromatography). After cooling at room temperature, change the reflux device to a vacuum distillation device, collect fractions at 58-68°C (50KPa), and recover 276g of N,N-dimethylformamide, with a recovery rate of 92%. Add 300g of water to dissolve the salt formed in the reaction process to the underpressure distillation residue, let it stand for layers, separate the organic phase, dry it with anhydrous sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com