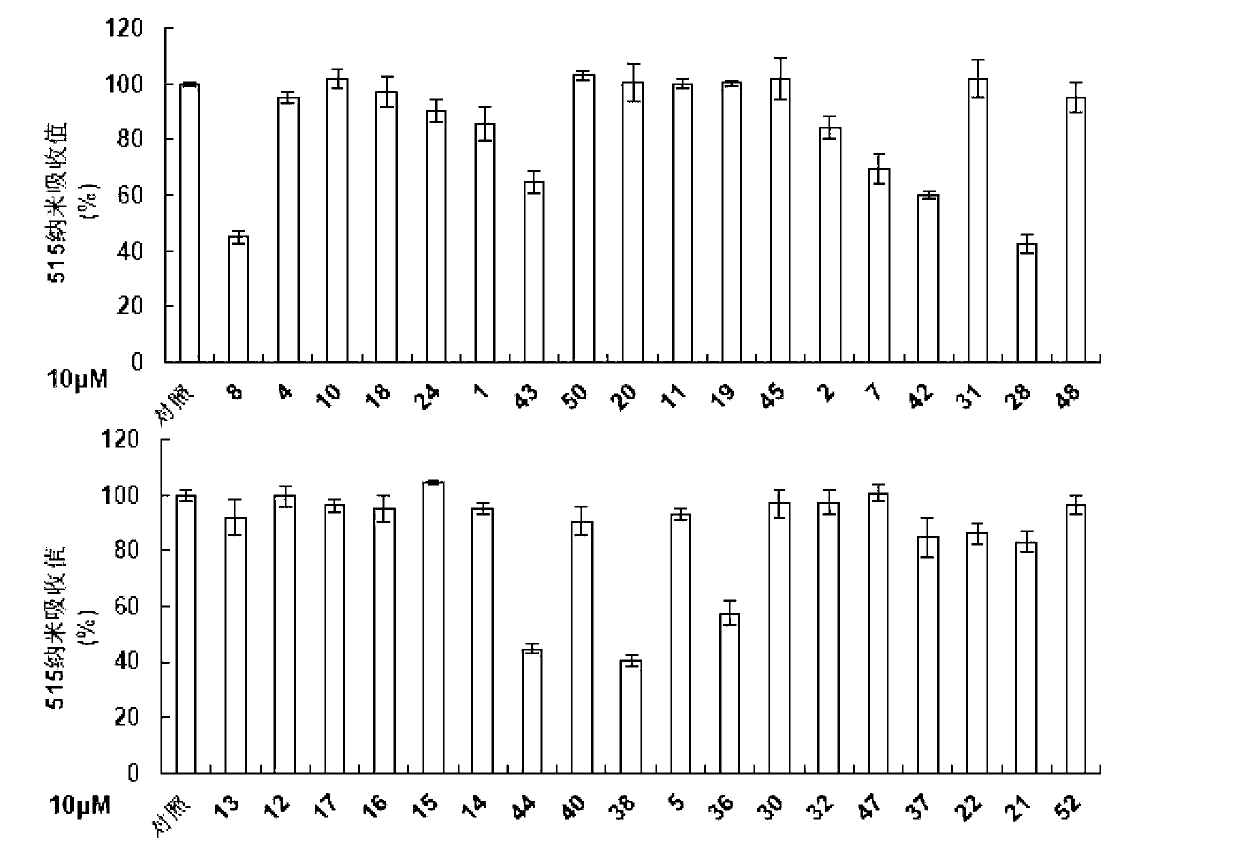
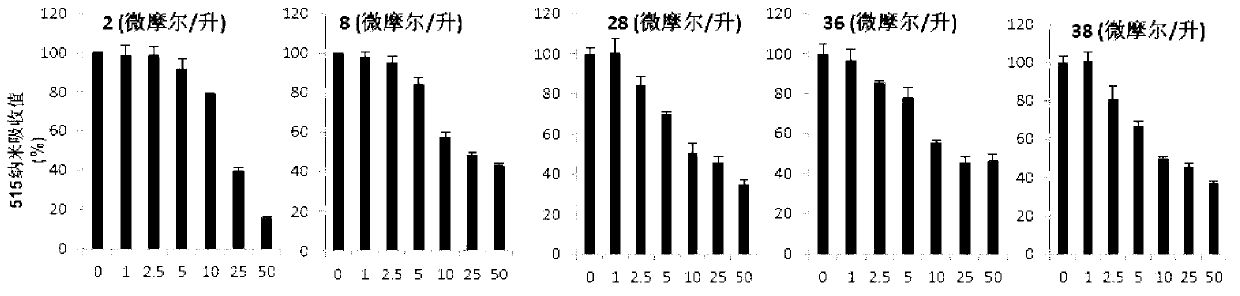
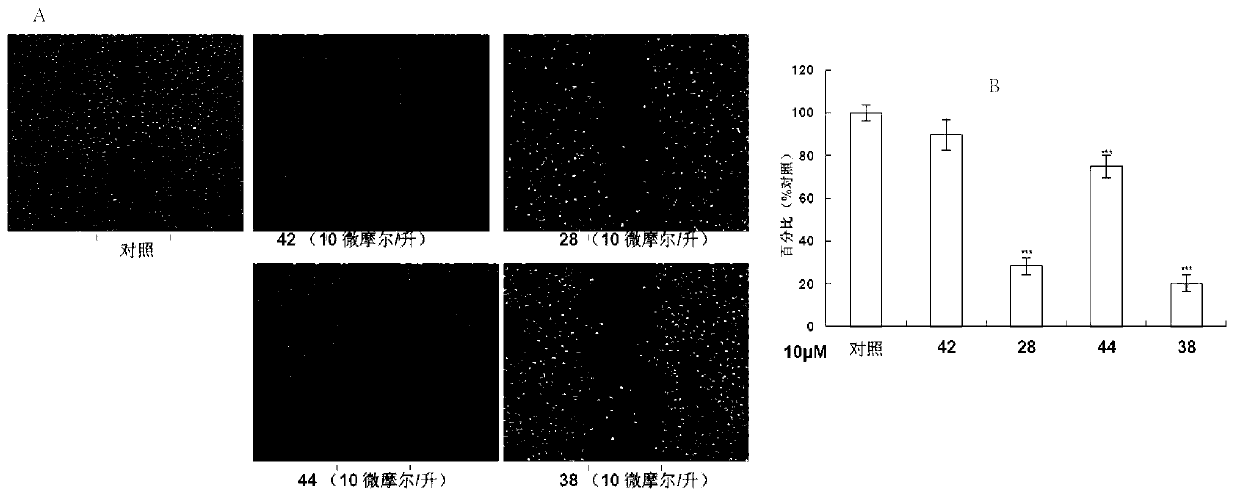
Application of aromatic alkanoyl tetrahydro-beta-carboline and derivative thereof in treatment of cancer
A technology of aryl alkanoyl tetrahydro and aryl, which is applied in the field of related diseases, can solve the problems of unsatisfactory physical and chemical properties of compounds, restrictions on the research of compound druggability, and unclear molecular mechanism of action of compounds, etc., to achieve improvement of pharmacokinetic properties , Excellent therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Embodiment one: the preparation of each compound
Embodiment 1-1
[0118] Example 1-1, Synthesis of N-phenylacetyl-(6-trifluoromethyl)-1,3,4,9-tetrahydro-1H-β-carboline (NTC001)
[0119]Take 4-trifluoromethylphenylhydrazine hydrochloride (425mg, 2mmol) in 14mL alcohol and water (volume ratio is 5 / 1), slowly add 4-chlorobutyraldehyde dimethyl acetal (367mg, 2.2 mmol), then warming and flowing for 5-18 hours. Complete, remove most of the solvent under reduced pressure, then adjust the pH value of the solution to 12, extract three times with ethyl acetate, combine the organic phases, dry with anhydrous sodium sulfate, and distill off the solvent under reduced pressure to obtain the intermediate 5-trifluoroform Tritryptamine (crude product 464mg).
[0120] 5-Trifluoromethyltryptamine (464mg, 2.0mmol) was dissolved in 3mL of glacial acetic acid, and 37% formaldehyde solution (45μL, 0.60mmol) was added dropwise under the protection of nitrogen to complete the addition and react overnight. Most of the acetic acid was evaporated under reduced press...
Embodiment 2-52
[0122] Preparation of NTC002-52 carboline compounds shown in Example 2-52 and Table 1 (see reference below for specific process)
[0123]
[0124]
[0125]
[0126]
[0127]
[0128]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com