Methods for treating methotrexate-resistant disorders with 10-propargyl-10-deazaaminopterin
A technology of methotrexate, propargyl, applied in the field of reduction, able to solve problems such as treatment failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
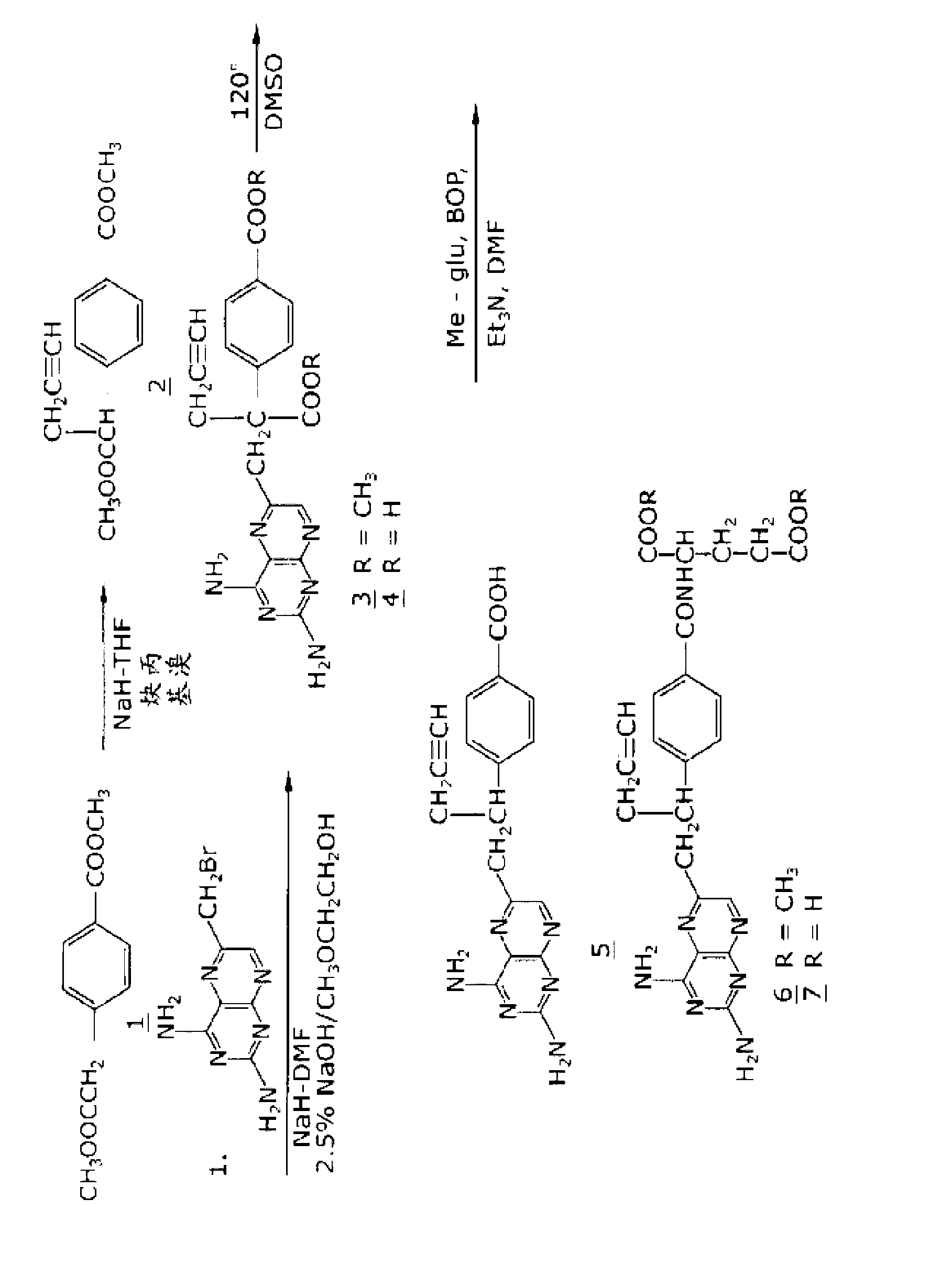
[0063] figure 1 A synthetic scheme suitable for the preparation of 10-propargyl-10-deazaminopterin is shown. A mixture of 60% NaH in oil (1.06 g, 26.5 mmol) in 18 mL of sieved dehydrated THF was cooled to 0 °C. With liter dimethyl terephthalate (5.0g, 24mmol., figure 1 The cold mixture was treated with a solution of compound 1) in anhydrous THF (7 mL), and the mixture was stirred at 0 °C for 1 h. Propargyl bromide (26.4 mmol) was added, and the mixture was stirred at 00°C for a further 1 hour, then at room temperature for 16 hours. The resulting mixture was treated with 2.4 mL of 50% acetic acid, then poured into 240 mL of water. The mixture was extracted with ether (2 x 150 mL). The ether extracts were combined and passed through Na 2 SO 4 Dry and concentrate to an orange-yellow oil. Chromatography on silica gel (600 mL 230-400 mesh) eluting with cyclohexane-EtOAc (8:1) gave the product α-propargyl dimethyl terephthalate (compound 2) as a white solid ( 4.66), the prod...
Embodiment 2
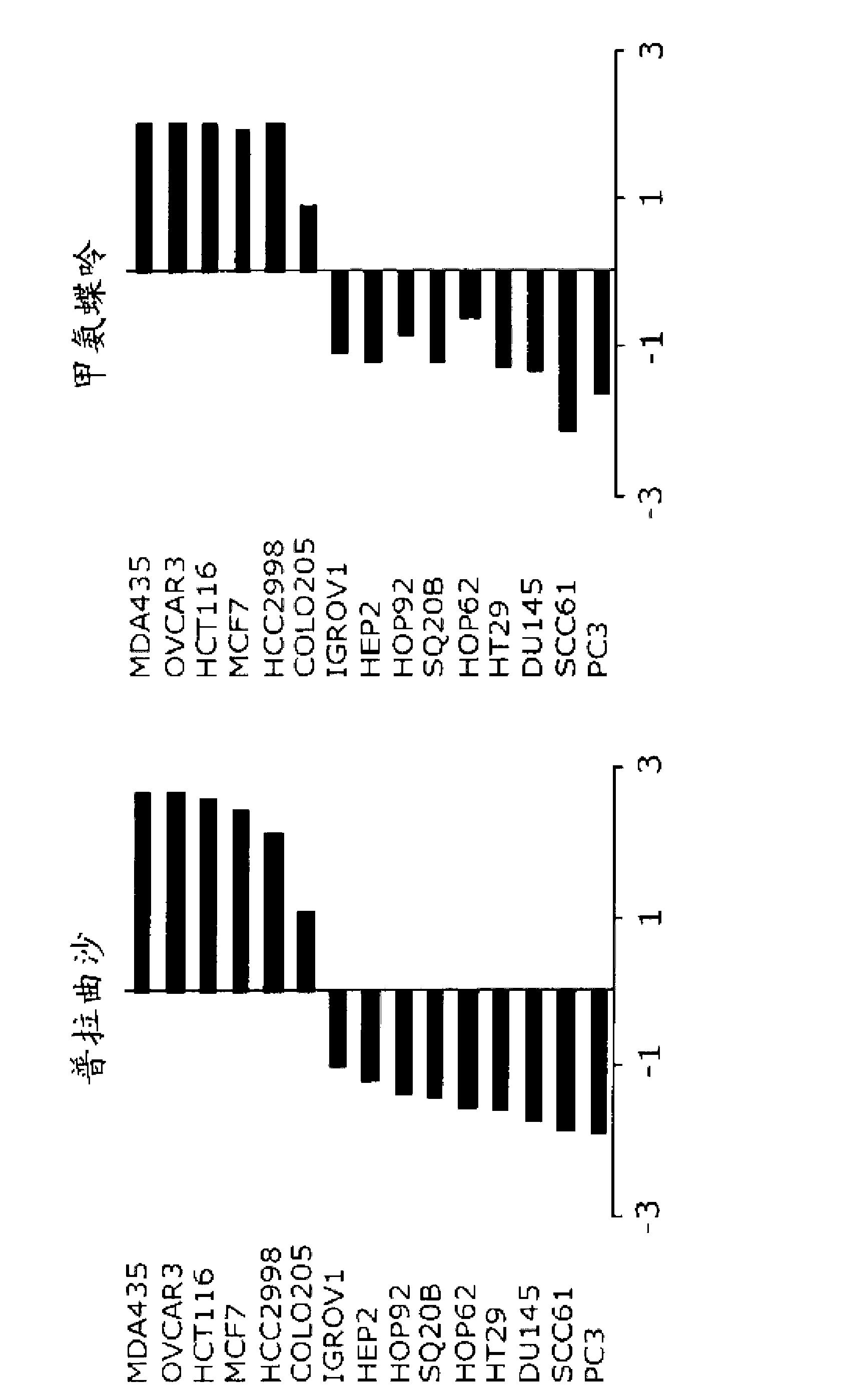
[0071] In order to investigate the activity of 10-propargyl-10-deazaminopterin on different solid tumor types, the cell lines of 15 human solid tumor cell lines were studied against 10-propargyl-10-deazaminopterin. Sensitivity to Toxic Activity.
[0072] Materials and methods: cell lines
[0073] A panel of human colon cancer (HT29, HCT116, COLO205, HCC2998), breast cancer (MCF7, MDA-MB-435), lung cancer (HOP62, HOP92), ovarian cancer (OVCAR3, IGROV1), prostate cancer (DU145, PC3) and head and neck cancer Carcinoma (SCC61, HEP2, SQ20B) cell lines were purchased from ATCC (Rockville, MD) and National Cancer Institute collections. Supplemented with 10% fetal bovine serum, 2mM glutamine, 100 units ml -1 Penicillin and 100 μM ml -1 Cells were grown as monolayers in RPMI medium with streptomycin.
[0074] Cytotoxicity assay
[0075] All data generated are the result of three separate experiments performed in duplicate. Cell viability was determined using the MTT assay perform...
Embodiment 3
[0091] Generation of 10-propargyl-10-deazaminopterin and methotrexate resistant cell lines.
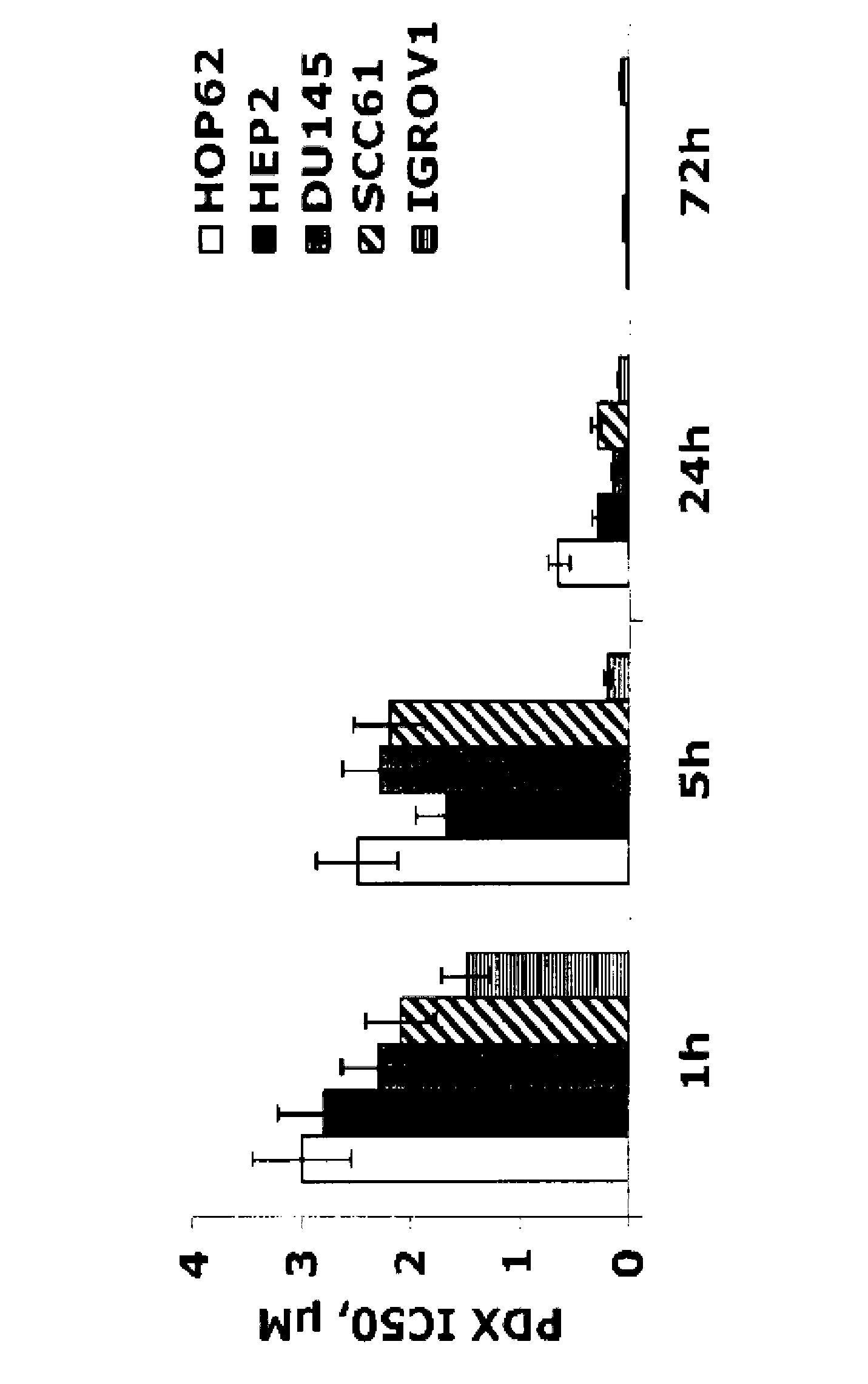
[0092] To characterize predictors of the antiproliferative effects of 10-propargyl-10-deazaminopterin, parental DU145 and HEP2 cells were exposed to increasing concentrations of 10-propargyl-10 over a period of 6 months, respectively. - Deazaminopterin to generate the cell lines DU-PDX and HEP-PDX. The resulting DU-PDX and HEP-PDX cells were at most 1 / 200 and 1 / 500 as sensitive to 10-propargyl-10-deazaminopterin as the parental cells. Resistant cells retained their drug resistance after 5 passages in drug-free medium, indicating the stability of these cell lines.
[0093] To compare the mechanisms of 10-propargyl-10-deazaminopterin and methotrexate resistance, the cell line DU-MTX was generated from parental DU145 and HEP2 cells by exposure to stepwise increasing concentrations of methotrexate and HEP-MTX. DU-MTX and HEP-MTX displayed resistance to methotrexate and 10-propargyl-10-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com