Apparatus and kit for encapsulating at least one compound for therapeutic and/or diagnostic use in erythrocytes
一种红细胞、化合物的技术,应用在药物的器械、其他医疗器械、微型胶囊等方向,能够解决犯错、耗时操作人员、设备不能适当地实施等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
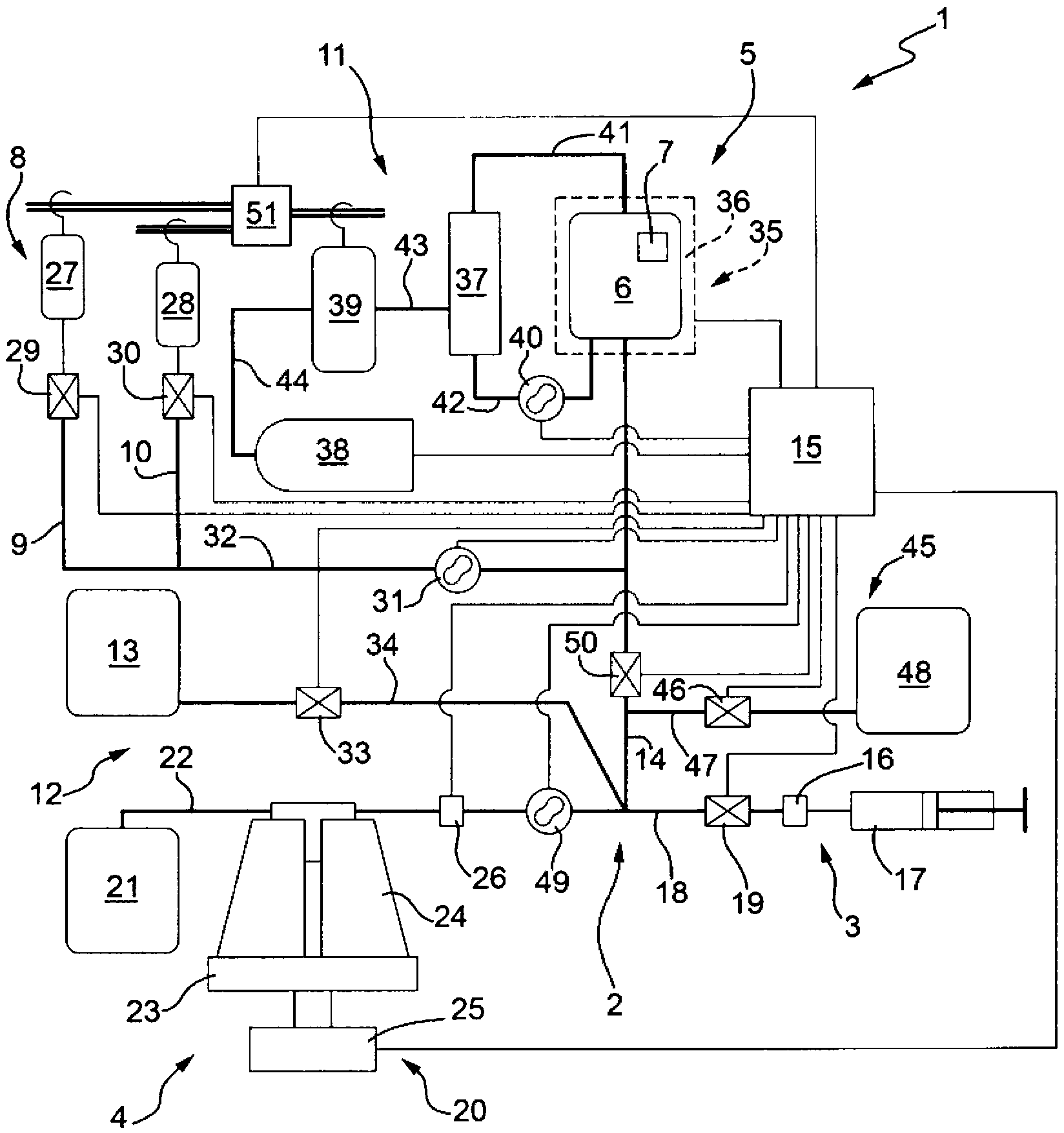
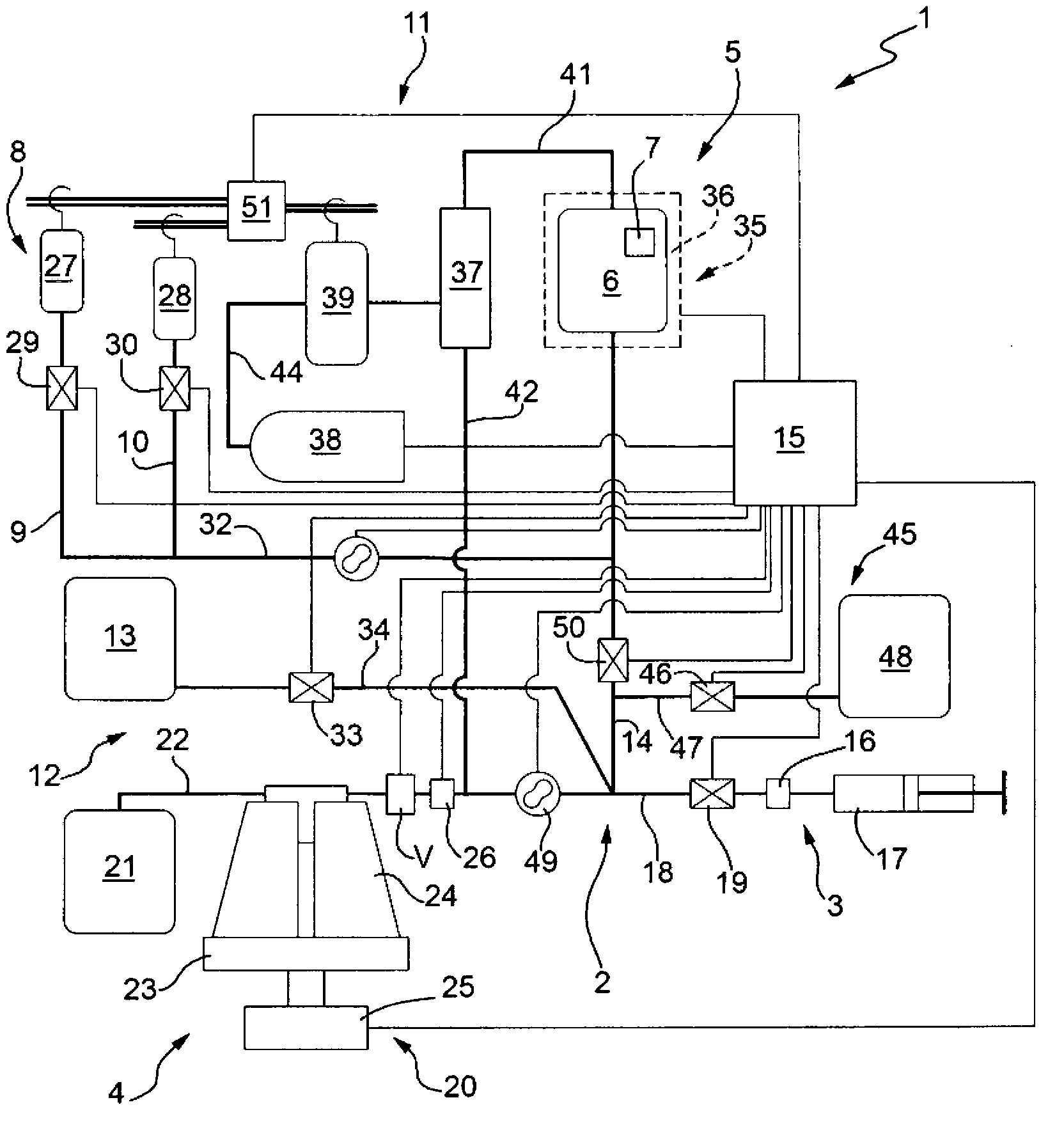
[0161] This example discloses figure 1 operation test of the equipment. The method used hereinafter follows the indications of the operating instructions of the above-mentioned device 1 with respect to the first aspect of the invention.
[0162] Eight experiments loaded with dexamethasone sodium phosphate (500 mg per procedure) were performed in human red blood cells from 50 ml of whole blood from healthy blood donors.
[0163] The materials used for the tests were:
[0164] - Figure 4 s installation;
[0165] - Figure 9 s installation;
[0166] - hypotonic solution 1 (400ml, osmolality 180mOsm / Kg), hypotonic solution 2 (200ml, osmolality 120mOsm / Kg);
[0167] - Resealing hypertonic solution (PIGPA) (about 33mM NaH 2 PO 4 about 1.606M KCl; about 0.194M NaCl; about 0.1M inosine; about 5mM adenine; about 20mM ATP; about 0.1M glucose; about 0.1M pyruvate; and about 4mM MgCl 2 ) (7ml, 2500-3800mOsm / Kg).
[0168] - Dexamethasone sodium phosphate aqueous solution, 500mg / ...
Embodiment 2
[0178] superparamagnetic nanoparticles
[0179] Superparamagnetic nanoparticles are already available and used as contrast agents for magnetic resonance imaging (MRI). However, once infused into the bloodstream by intravenous injection, the nanoparticles are rapidly coated by the plasma components of the blood, a process called opsonization that establishes the vulnerability of the nanoparticles themselves to the body's main defense system, the mononuclear phagocyte system. Identify the key to the assurance. Thus, the encapsulation of superparamagnetic nanoparticles in human erythrocytes has been achieved by the device of the present invention to avoid their rapid clearance from the blood circulation and thus obtain a wider imaging time range in intravascular magnetic resonance applications ( PCT / EP07 / 06349, Delivery of contrasting agents for magnetic resonance imaging). For example, having passed the procedure disclosed above and as an object of the present invention such...
Embodiment 3
[0182] Contrast agent Indocyanine Green (ICG)
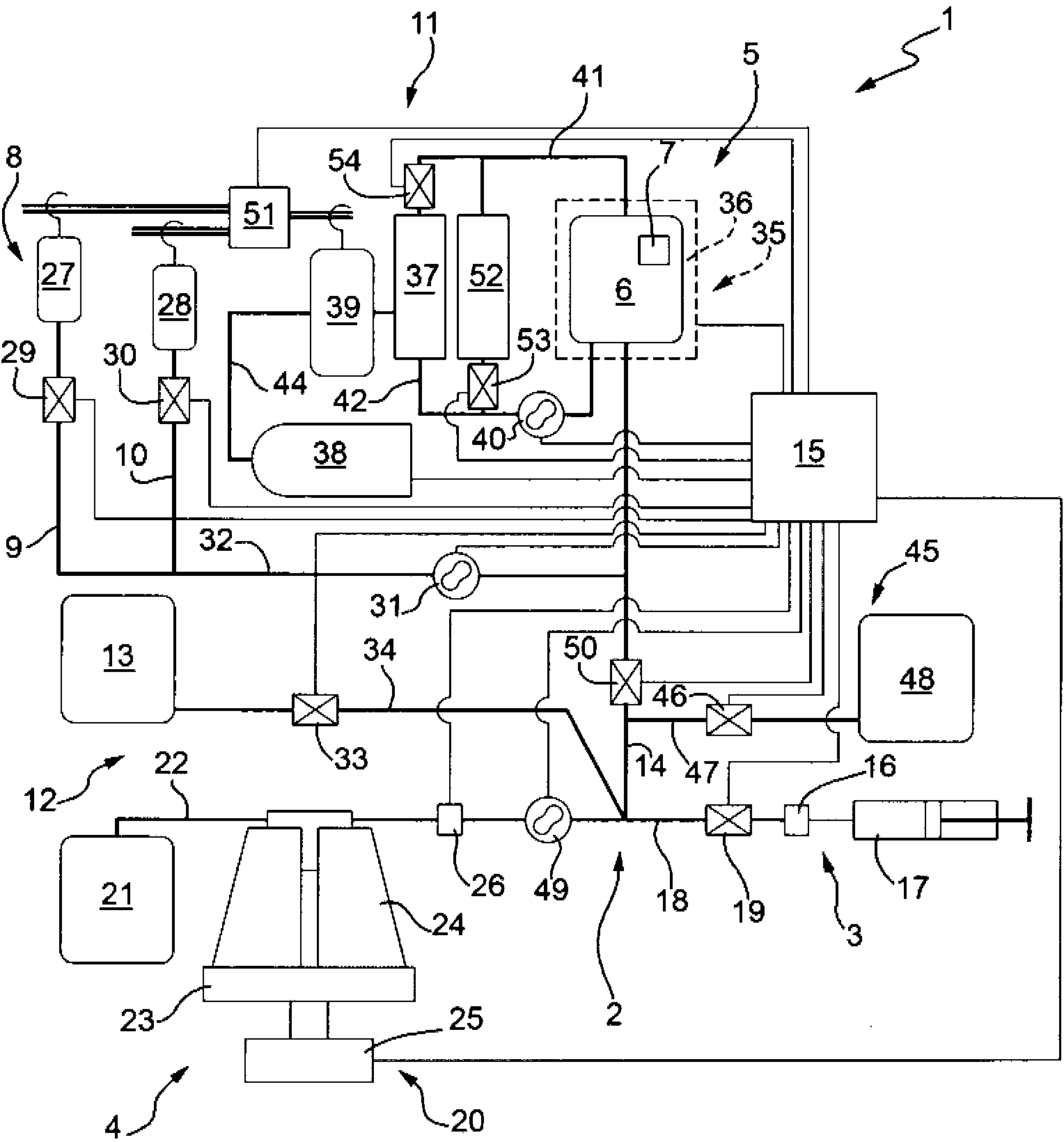
[0183] Another application of the innovative technology involving the delivery of exogenous molecules into red blood cells is represented by the encapsulation of contrast agents to be used in fluorescein angiography or other optical and / or fluorescent detection methods. For example, using figure 2 The device shown represents the results obtained for the encapsulation of the contrast agent indocyanine green (ICG). This is proposed as a new strategy for visualization and / or photocoagulation of choroidal neovascularization in retinal degeneration and vascular disease for diagnostic and therapeutic purposes.
[0184] ICG is an FDA-approved tricarbocyanine-containing infrared (IR) contrast agent for diagnostic use in visualizing retinal vascularity and for photodynamic therapy. Its use as a contrast agent takes advantage of the fact that most biomolecules neither absorb nor emit light in the near-infrared region, resulting in int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com