Trifluoromethyl carbonyl isoindigo derivative and synthesis method for same
A technology of trifluoromethylcarbonylisoindigo and fluoromethylcarbonylisoindigo, which is applied in the field of trifluoromethylcarbonylisoindigo derivatives and their synthesis, and can solve the undiscovered synthesis report of trifluoromethylcarbonylisoindigo derivatives and other problems, to achieve the effect of lowering the LUMO energy level, simple synthesis method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
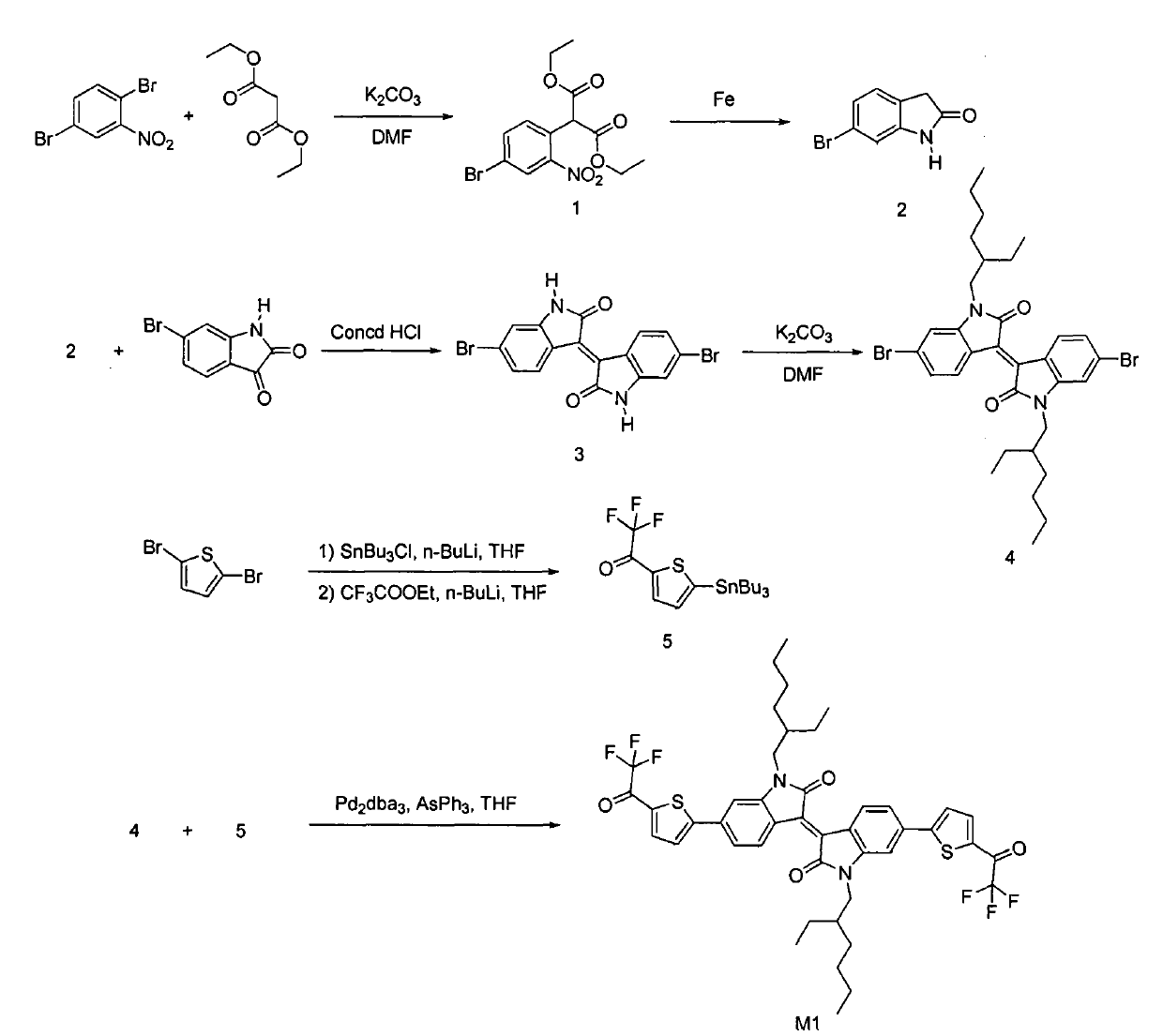
[0018] This example provides a soluble trifluoromethylcarbonyl isoindigo derivative, its structural formula is shown in Table 1, and its synthetic route can be found in figure 1 .
[0019] Table 1
[0020]
[0021] Preparation of compound M1
[0022] The synthetic method of described a kind of trifluoromethylcarbonyl isoindigo derivative (M1) comprises the steps:
[0023] (a) Synthesis of intermediate compound 4
[0024] The structural formula of intermediate compound 4 is:
[0025]
[0026] like figure 1 As shown, compound 4 was prepared by the literature method, and its detailed preparation method can be found in the literature "Mei, J.; Graham K.R.; Stalder, R.; Reynolds, J.R. Synthesis of Isoindigo-Based Oligothiophenes for Molecular Bulk Heterojunction Solar Cells, Org. Lett ., 4, 660-663(2010).》
[0027] (b) Synthesis of intermediate compound 5
[0028] The structural formula of intermediate compound 5 is:
[0029]
[0030] Its preparation method compri...
Embodiment 2
[0037] Embodiment 2, the ultraviolet absorption spectrum, electrochemical property, heat resistance of isoindigo derivative
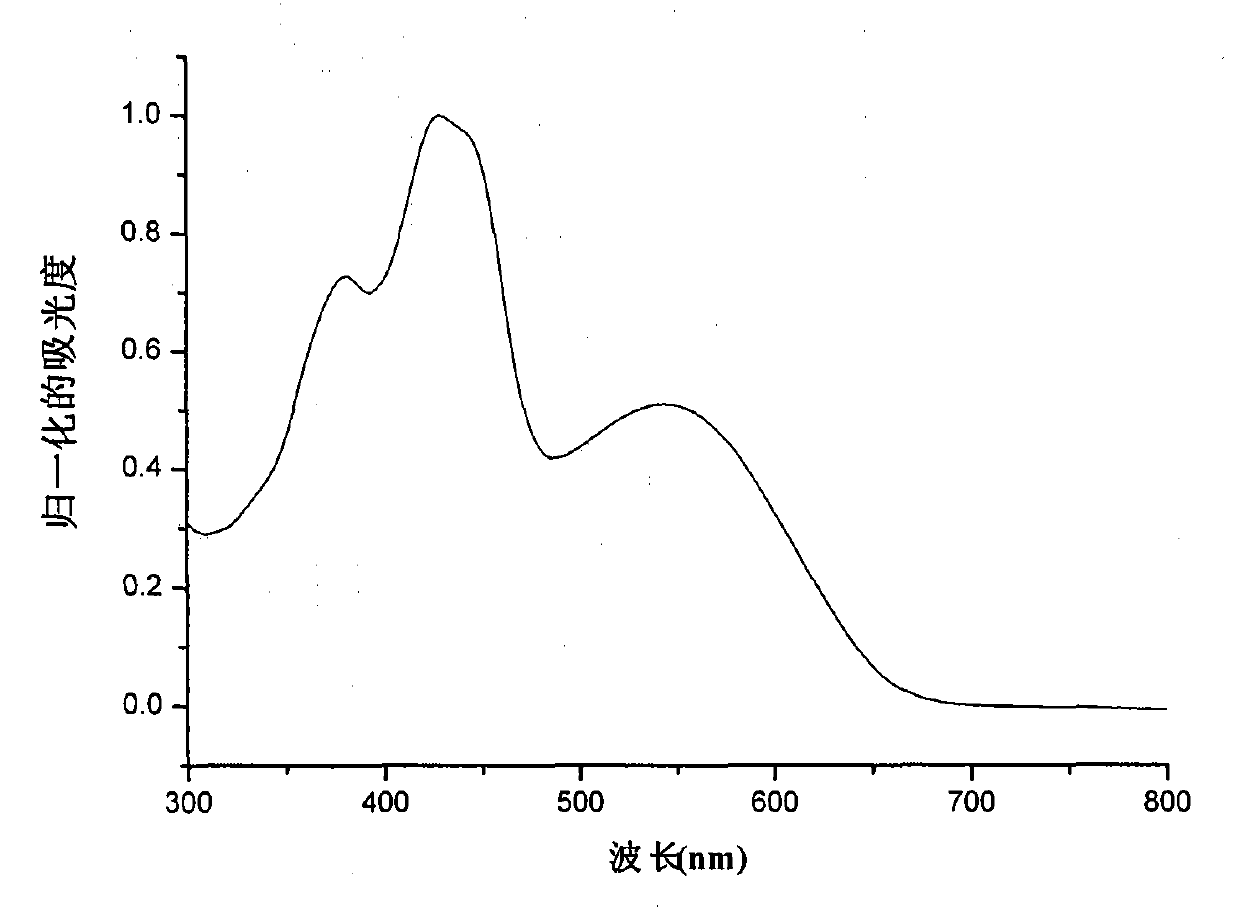
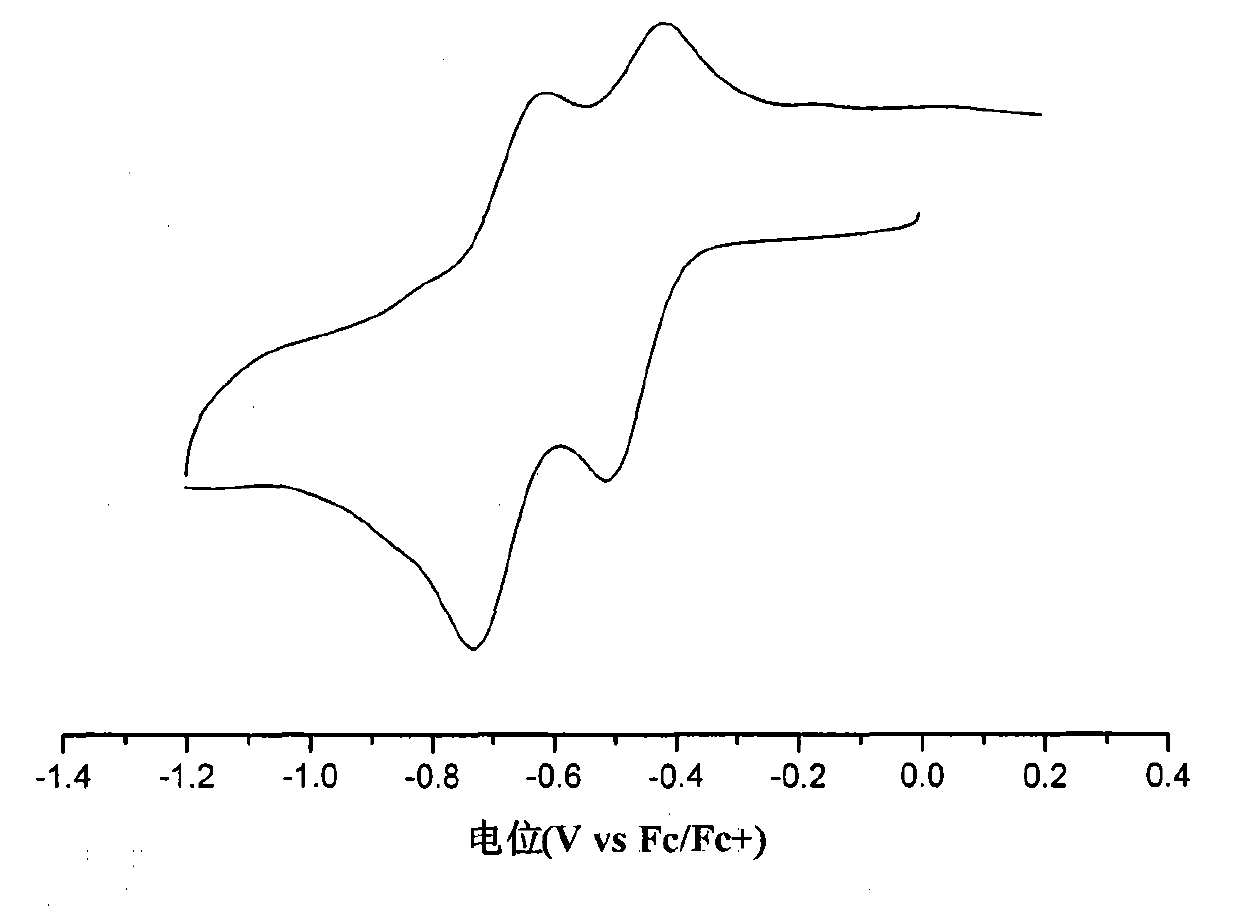
[0038] figure 2 The ultraviolet absorption spectrum of isoindigo derivative M1 in chloroform and thin film is given, the maximum absorption peak position of its thin film is around 435nm, and the optical band gap is 1.91eV. image 3 The cyclic voltammetry curve of isoindigo derivative M1 is given. The cyclic voltammetry test is carried out on the computer-controlled CHI610D telephone line analyzer, using the traditional three-electrode test system, the platinum electrode is the working electrode, the silver / silver ion electrode is the reference electrode, and the electrolyte is tetra-n-butyl hexafluorophosphoric acid Ammonium in acetonitrile solution (0.1M), scan speed is 100mV / s, with ferrocene as reference. The oxidation potential of ferrocene measured under this system is 0.05eV, because the energy level of ferrocene under vacuum conditions is 4.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com