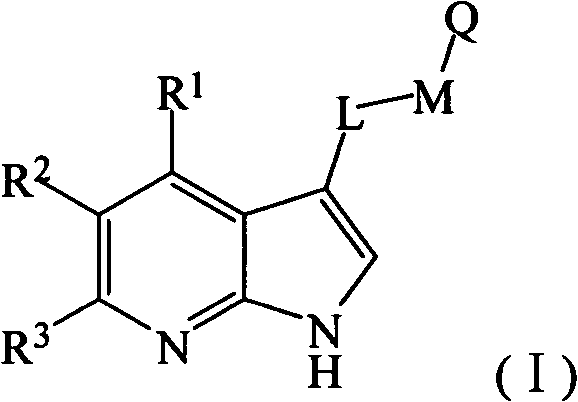
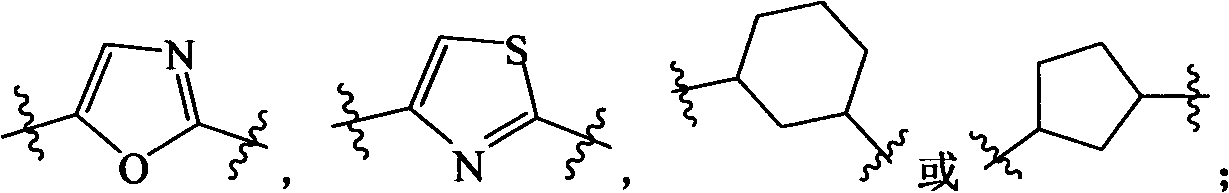
Heterocycle substituted pyrido-pyrrole kinase inhibitor
A technology of heterocyclic group and cycloalkyl group, which is applied in the direction of medical preparations containing active ingredients, urinary system diseases, organic active ingredients, etc., and can solve problems such as poor selectivity and insufficient activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0117] (2) Preparation of Intermediate TM 2
[0118] Dissolve the intermediate TM 1 (1 equivalent) in a mixed solution of an appropriate amount of methanol and water, add NaOH (3 equivalents), and react under the protection of nitrogen. After the reaction is completed for 6 hours, the reaction solution is spin-dried, and the pH is adjusted to 3 with dilute hydrochloric acid. . Dichloromethane was added to the mixture, extracted, separated, and the organic phase was anhydrous Na 2 SO 4 Dry and evaporate to dryness to obtain the intermediate TM 2.
[0119] (3) Preparation of Intermediate TM 3
[0120] The obtained intermediate TM 2 (1 equivalent) was dissolved in an appropriate amount of toluene, and then SOCl was added dropwise 2 (6 equivalents), reflux reaction at 100°C, after the reaction is complete, the reaction solution is spin-dried to obtain the crude product of intermediate TM 3, which is set aside.
[0121] (4) Preparation of Intermediate TM 4
[0122] The starti...
Embodiment 1
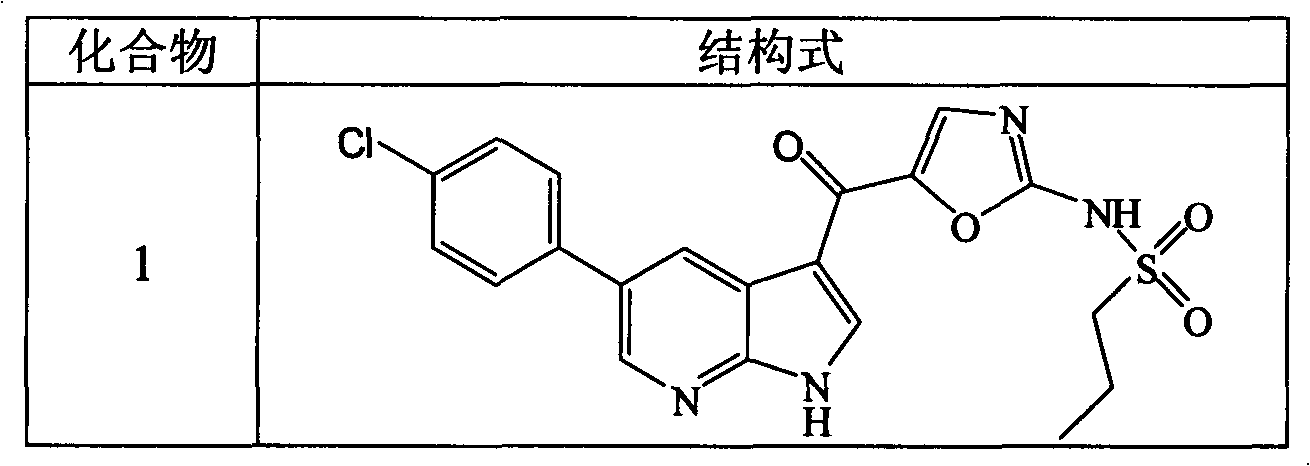
[0143] Example 1N-(5-(5-(4-chlorobenzene)-1H-pyrrole[2,3-b]pyridine-3-carbonyl)oxazol-2-yl)propane-1-sulfonamide (compound 1) Preparation
[0144]
[0145] (1) Preparation of intermediate TM 1-1
[0146] Under ice bath, the starting material SM 1 (2.34 g, 15.0 mmol) was dissolved in 50 ml CH 2 Cl 2 , and pyridine (3.8g, 50mmol) was added dropwise, and then 1-chloropropanesulfonyl chloride (2.8g, 15mmol) was added dropwise to the reaction solution. After the addition was complete, it was moved to room temperature and stirred overnight. After the reaction was completed, the reaction solution was spin-dried, washed with water and brine successively, and washed with anhydrous Na 2 SO 4 After drying, filtering, and column chromatography, intermediate TM 1-1 was obtained, 2.79 g, yield 71%.
[0147] (2) Preparation of Intermediate TM 1-2
[0148] The intermediate TM 1-1 (1.31g, 5.0mmol) was dissolved in a mixed solution of 40ml of methanol and water, NaOH (0.6g, 15.0mmol)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ec50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com