Dolasetron mesylate containing injection, as well as preparation method and quality control method thereof
A technology for dolasetron mesylate and injections, which is applied in the field of injections containing dolasetron mesylate and its preparation, and can solve problems such as interruption, pain of patients, and cessation of anti-tumor treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the impact of polyhydroxy compound on the stability of dolasetron mesylate injection
[0044] Prescription 1 Prescription 2 Prescription 3 Prescription 4 Prescription 5 Prescription 6 Prescription 7 Prescription 8 dolasetron mesylate 20g 20g 20g 20g 12.5g 12.5g 12.5g 12.5g Mannitol - 38.2g 33.0g 30.0g - 38.2g 30.0g 30.0g Sorbitol - - 5.0g - - - 7.5g - xylitol - - - 7.5g - - - 5.0g Add water for injection to 1000ml 1000ml 1000ml 1000ml 1000ml 1000ml 1000ml 1000ml
[0045]According to the above prescription, take the mannitol, sorbitol or xylitol of the prescription amount respectively, after dissolving completely with the water for injection of 70% of the prescription amount cooled to room temperature, add the dolasetron mesylate of the prescription amount and stir to dissolve ( Concentrated formulation), add 0.05% (W / V) of concentrated formulation volume of medici...
Embodiment 2
[0064] Embodiment 2: the influence of pH value on the stability of dolasetron mesylate injection
[0065] Prescription 2 Prescription 6 dolasetron mesylate 20.0 g 12.5g Mannitol 38.2 g 38.2g Water for Injection Add to 1000ml Add to 1000ml
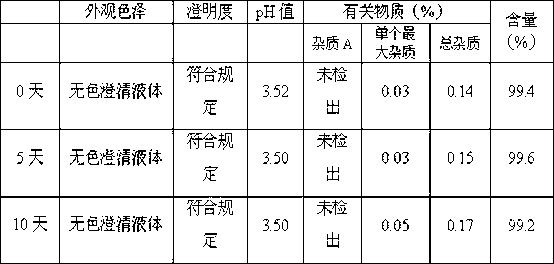
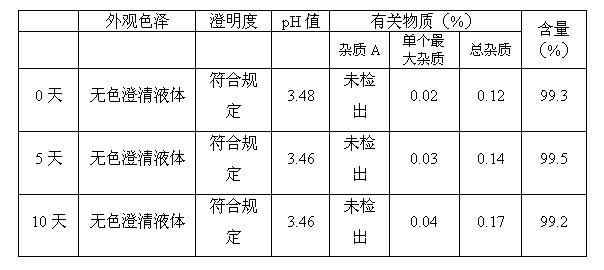
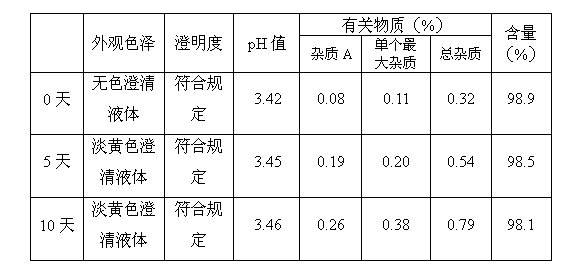
[0066] According to the above prescription, weigh the prescribed amount of mannitol respectively, completely dissolve it with 70% of the prescribed amount of water for injection cooled to room temperature, add the prescribed amount of dolasetron mesylate and stir to dissolve (concentrated formulation), add the concentrated formulation 0.05% of the volume (W / V) of medicinal activated carbon was stirred evenly, and after standing for 10 minutes to absorb, filter and decarbonize; add water for injection to 95% of the total amount, stir evenly, and adjust the pH value to 0.1mol / L hydrochloric acid solution respectively. 3.0, 3.5, 4.0, 4.5, add water for injection to the full amount, stir evenly; fine fil...
Embodiment 3
[0074] Feeding prescription:
[0075] dolasetron mesylate 20.0 g Mannitol 38.2 g Water for Injection Add to 1000ml
[0076] Weigh the prescribed amount of mannitol, completely dissolve it with 70% of the prescribed amount of water for injection cooled to room temperature, add the prescribed amount of dolasetron mesylate and stir to dissolve (concentrated formulation), add 0.05% of the concentrated formulation volume ( W / V) of medicinal activated carbon, stir evenly, let it stand for 10 minutes for adsorption, filter and decarbonize; add water for injection to 95% of the total amount, stir evenly, adjust the pH to 3.0 with 0.1mol / L hydrochloric acid solution, and add water for injection to Stir the whole amount evenly; take a sample to measure its properties, pH value, content and bacterial endotoxin. After passing the test, fine filter, fill with nitrogen, fill, melt seal, and sterilize with damp heat at 121°C for 12 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com