Method for preparing (Y1-xEux)2(OH)5NO3.nH2O oversized rare-earth layered hydroxide compound particles
A technology of oxyhydroxide and 3·6H2O, which is applied in the field of material science, can solve the problems of limiting the size of stripped nanosheets and not obtaining LRH pure phase particles, and achieves the effect of easy stripping and simple technical solutions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Eu(NO 3 ) 3 ·6H 2 O and Y (NO 3 ) 3 ·6H 2 O is mixed with ammonium nitrate according to the molar ratio Y / Eu=19:1. The amount of ammonium nitrate added is based on molar ratio. Stir under pressure to become transparent, and prepare a solution with a total concentration of rare earth element ions of 0.10mol / L, then add ammonium hydroxide while stirring, and adjust the pH of the solution to 6.5 to obtain a suspension;
[0031] The above suspension was moved to a reaction kettle, and hydrothermally reacted at 200 o C for 24 hours;
[0032] After the reaction, the reactor was taken out, cooled to room temperature naturally, and the reaction product was centrifuged, cleaned, and dried at 60°C to obtain white powdery particles (Y 0.95 Eu 0.05 ) 2 (OH) 5 NO 3 · n h 2 O, n =1.5-1.8.
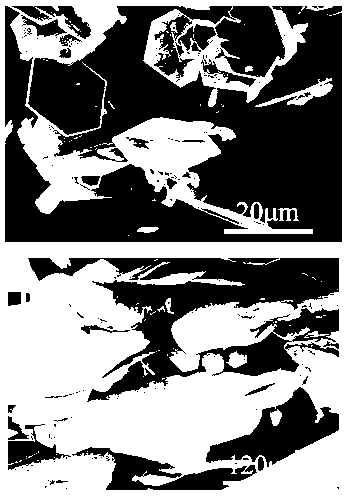
[0033] The obtained white powder particles have a side length of 20 μm, such as figure 1 (a) shown.
Embodiment 2
[0035] Eu(NO 3 ) 3 ·6H 2 O and Y (NO 3 ) 3 ·6H 2 O is mixed with ammonium nitrate according to the molar ratio Y / Eu=19:1, the amount of ammonium nitrate added is based on molar ratio, ammonium nitrate: (Eu+Y) ion=150:1, add deionized water, at room temperature Stir under pressure to become transparent, and prepare a solution with a total concentration of rare earth element ions of 0.10mol / L, then add ammonium hydroxide while stirring, and adjust the pH of the solution to 6.5 to obtain a suspension;
[0036] The above suspension was moved to a reaction kettle, and hydrothermally reacted at 200 o C for 24 hours;
[0037] After the reaction, the reactor was taken out, cooled to room temperature naturally, and the reaction product was centrifuged, cleaned, and dried at 60°C to obtain white powdery particles (Y 0.95 Eu 0.05 ) 2 (OH) 5 NO 3 · n h 2 O, n =1.5-1.8.
[0038] The obtained white powder particles have a side length of 300 μm, such as figure 1 (b) shown.
Embodiment 3
[0040] Eu(NO 3 ) 3 ·6H 2 O and Y (NO 3 ) 3 ·6H 2 O is mixed with ammonium nitrate according to the molar ratio Y / Eu=1:4, the amount of ammonium nitrate added is molar ratio, ammonium nitrate: (Eu+Y) ion=100:1, add deionized water, at room temperature Stir under pressure to become transparent, and prepare a solution with a total concentration of rare earth element ions of 0.08mol / L, then add ammonium hydroxide while stirring, and adjust the pH of the solution to 7.0 to obtain a suspension;
[0041] The above suspension was moved to a reaction kettle, and hydrothermally reacted at 120oC for 168 hours;
[0042] After the reaction finished, take out the reaction kettle, cool to room temperature naturally, the reaction product is through centrifugation, cleaning, and drying at 80oC to obtain white powdery particles (Y 0.2 Eu 0. 8 ) 2 (OH) 5 NO 3 · n h 2 O, n =1.5-1.8.
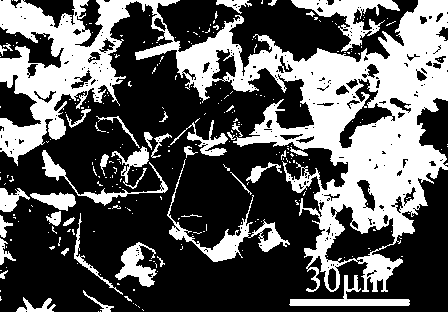
[0043] The obtained white powder particles have a side length of 10 μm, such as figure 2 As shown...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com