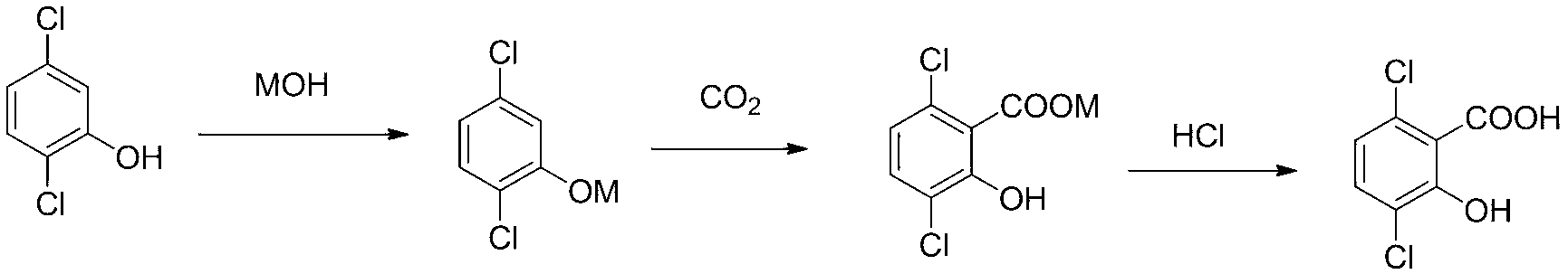
Preparation method of 3,6-dichloro-2-hydroxybenzoic acid
A technology of hydroxybenzoic acid and hydroxybenzoate, applied in the field of synthesis of 3,6-dichloro-2-hydroxybenzoic acid, which can solve the problems of reduced reaction yield and achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, a kind of preparation method of 3,6-dichloro-2-hydroxybenzoic acid, carries out following steps successively:
[0032] 1) Weigh 146g (0.83 mol) of 2,5-dichlorophenol (95% purity) in a 1000ml three-neck flask and dissolve it in 98g of KOH aqueous solution (mass concentration 48%, containing 0.84mol of KOH), and then add Mix 640ml of xylene as a solvent evenly, heat up to 142~145°C, dehydrate with a water separator until almost no water is evaporated, and obtain 2,5-dichlorophenoxide potassium solution.
[0033] 2), transfer the potassium 2,5-dichlorophenate solution obtained in the above step 1) to the autoclave, and then add 12gK 2 CO 3 and 14g of activated carbon as a co-catalyst, filled with CO in the autoclave 2 To 3Mpa, after being stabilized, empty the air, and continue the same operation for 3 times, so as to fully replace the air in the kettle; then fill with CO 2 to 5Mpa and heated; finally, control the reaction temperature to 145°C and the reac...
Embodiment 2
[0036] 14g of activated carbon in Example 1 was changed to 13.8g of recovered activated carbon (obtained in Example 1) + 0.2g of activated carbon, and the rest were the same as in Example 1. The result is:
[0037] 13.9 g of activated carbon was recovered and 73 g (0.437 mol) of 3,5-dichlorophenol was recovered, and the calculated recovery rate was 52.7%. 80 g (0.386 mol) of 3,6-dichloro-2-hydroxybenzoic acid solid was obtained, and the calculated yield was 46.5%. The calculated total yield of starting material and product was 99.2%.
Embodiment 3
[0039] Change the activated carbon 14g in Example 1 into 13.9g of recovered activated carbon (obtained in Example 2)+0.1g activated carbon, and the rest are the same as in Example 1. The result is:
[0040] 13.9 g of activated carbon was recovered and 72.1 g (0.432 mol) of 3,5-dichlorophenol was recovered, with a calculated recovery rate of 52.0%. 80.8 g (0.390 mol) of 3,6-dichloro-2-hydroxybenzoic acid solid was obtained, and the calculated yield was 47.0%. The total yield of starting material and product was calculated to be 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com