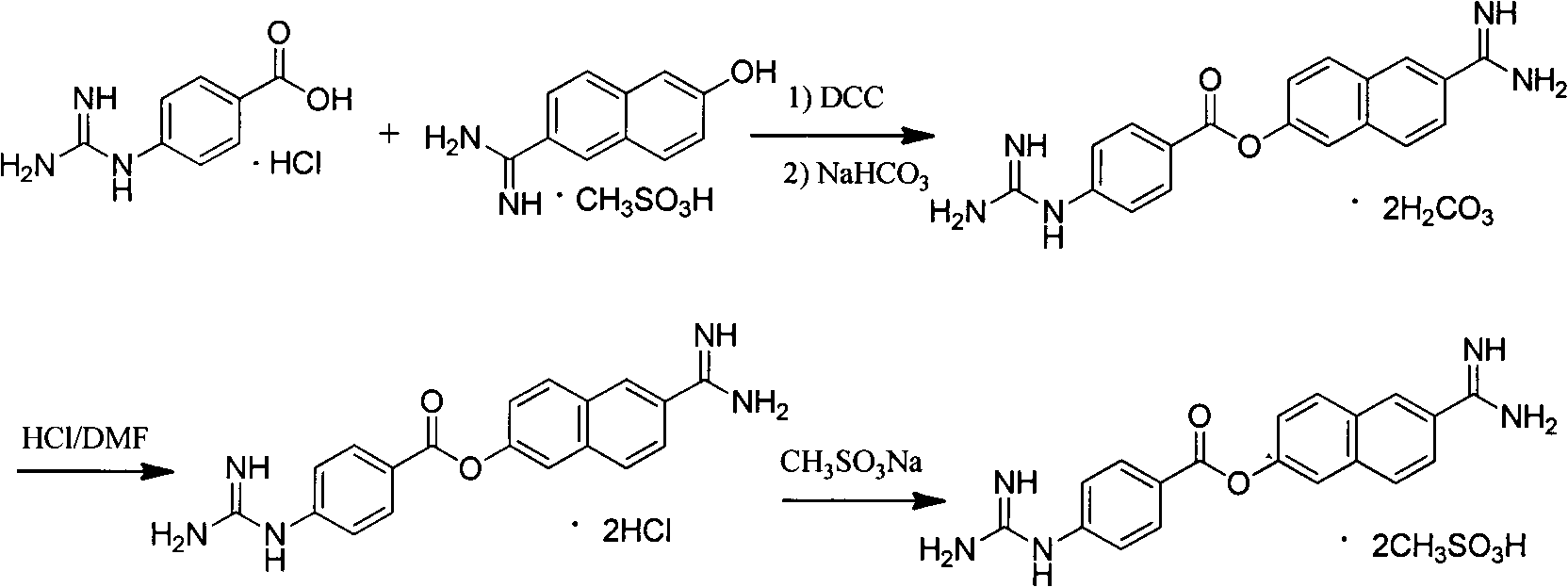
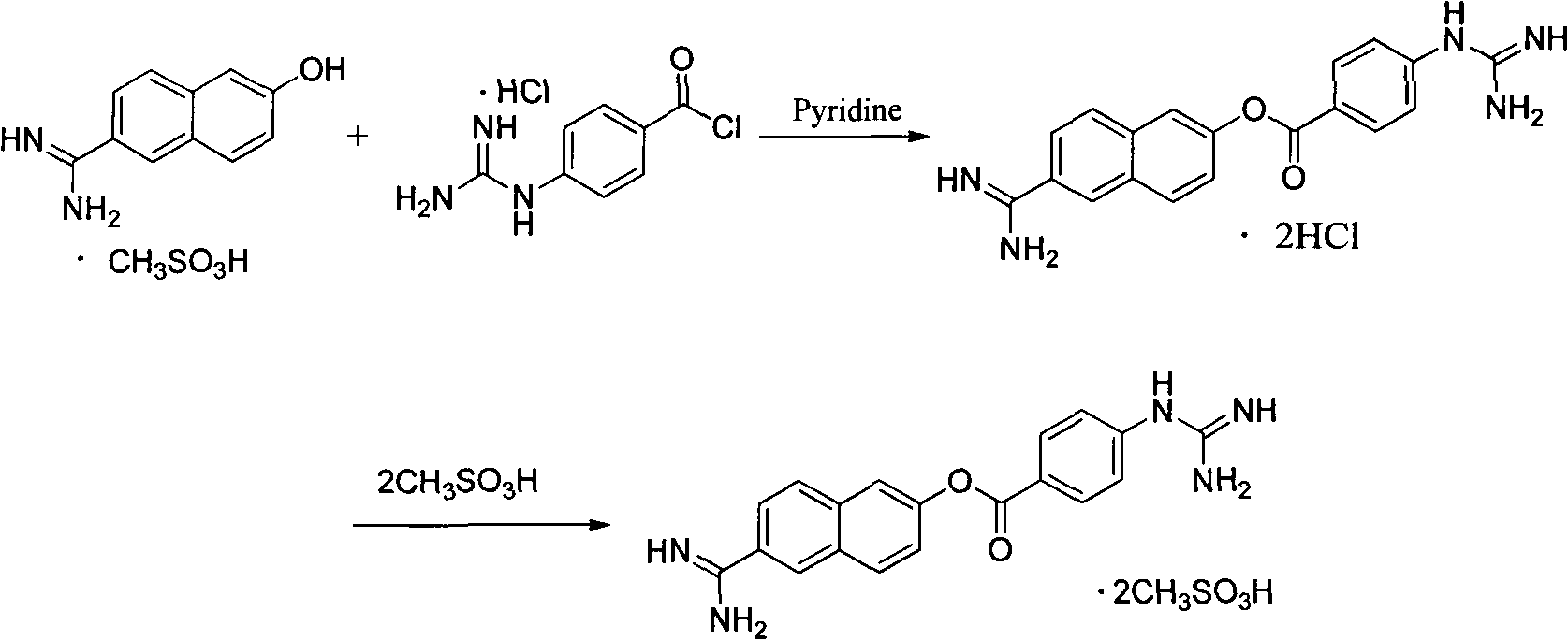
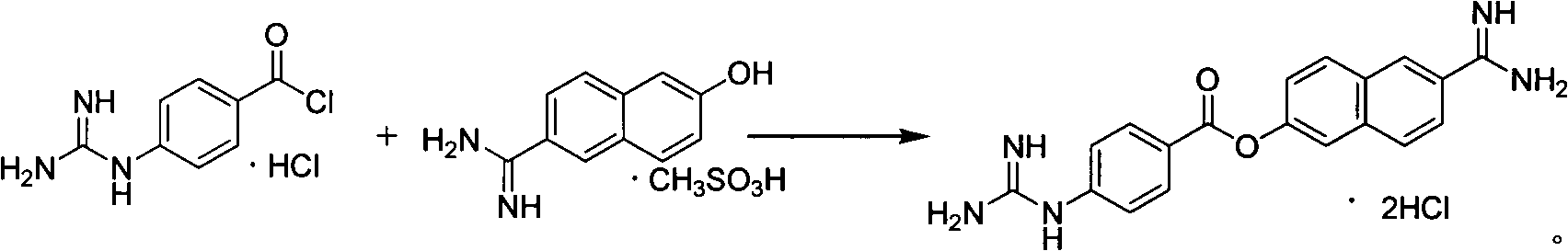
Method for preparing nafamostat hydrochloride and nafamostat mesylate
A technology of nafamostat mesylate and nafamostat, which is applied in the field of drug synthesis, can solve the problems of increased production cost and low yield of nafamostat sulfonate, and achieve high cost and good solid state , The effect of reducing the use of production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 nafamostat hydrochloride
[0032] 60 ml of pyridine was added to the reaction flask, and 6-amidino-2-naphthol methanesulfonate (28.23 g, 0.10 mol) was added under stirring. The temperature was lowered to 0-5°C in an ice-water bath, and p-guanidinobenzoyl chloride hydrochloride (28.09 g, 0.12 mol) was added in three batches. After feeding the materials, continue to keep the reaction at 0-5°C for 3h. After the reaction was completed, the temperature was naturally raised to room temperature, and the reaction was stirred for 10 h. After filtering, the filter cake was washed twice with 20ml of pyridine, and sucked dry. 29.65 g (HPLC: 93.5%) of a yellow solid was obtained. Melting point: Decompose at about 274°C. Yield: 70.90%.
Embodiment 2
[0033] The preparation of embodiment 2 nafamostat hydrochloride
[0034] 60 ml of pyridine was added to the reaction flask, and 6-amidino-2-naphthol methanesulfonate (28.23 g, 0.10 mol) was added under stirring. The temperature was lowered to 0-5°C in an ice-water bath, and p-guanidinobenzoyl chloride hydrochloride (45 g, 0.18 mol) was added in three batches. After feeding the materials, continue to keep the reaction at 0-5°C for 2h. After the reaction was completed, the temperature was raised to 40° C., and the reaction was stirred for 8 hours. After filtering, the filter cake was washed twice with 20ml of pyridine, and sucked dry. 30.53 g (HPLC: 94.2%) of a yellow solid was obtained. Melting point: Decompose at about 274°C. Yield: 73.0%.
Embodiment 3
[0035] The preparation of embodiment 3 nafamostat hydrochloride
[0036] 60 ml of N,N-diisopropylethylenediamine was added to the reaction flask, and 6-amidino-2-naphthol methanesulfonate (29.12 g, 0.103 mol) was added under stirring. The temperature was lowered in an ice-water bath to -10-5°C, and p-guanidinobenzoyl chloride hydrochloride (72.43 g, 0.31 mol) was added in four batches. After the feed is finished, continue to react at -10~5°C for 5h. After the reaction was completed, the temperature was naturally raised to room temperature, and the reaction was stirred for 10 h. After filtering, the filter cake was washed twice with 20 ml of N,N-diisopropylethylenediamine and sucked dry. A yellow solid was obtained, about 30.78 g (HPLC: 91.8%). Melting point: Decompose at about 274°C. Yield: 71.00%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com