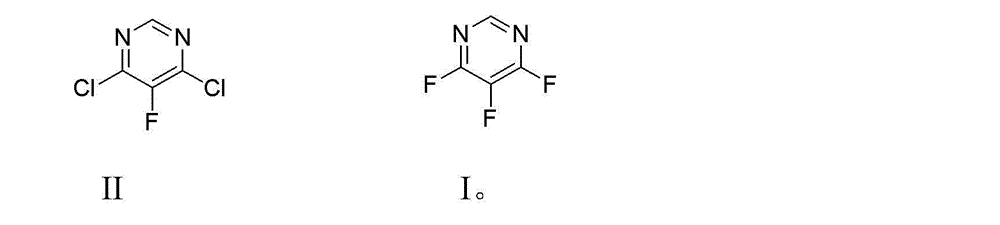
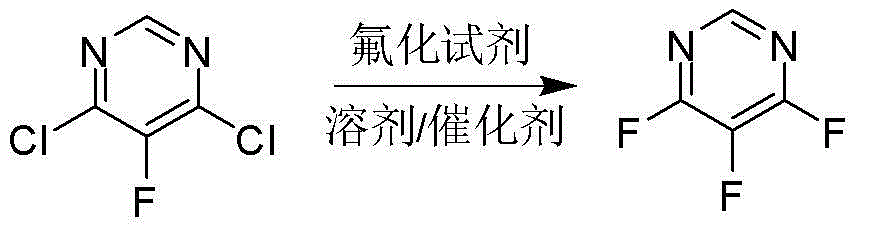
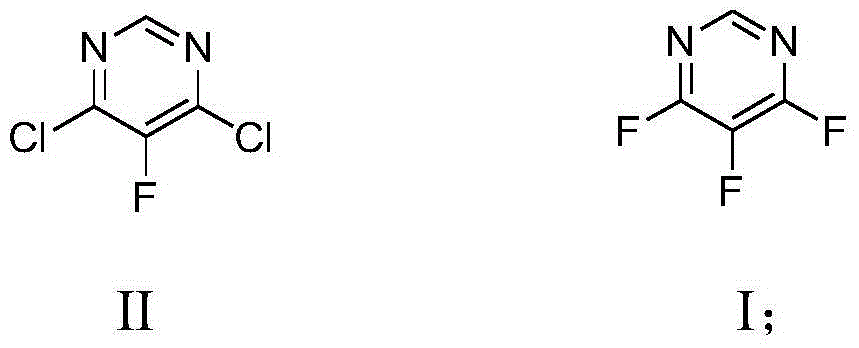Preparation method of 4,5,6-trifluoro-pyrimidine compound
A technology of trifluoropyrimidine and compounds, applied in the direction of organic chemistry, can solve the problems of harsh process conditions, unstable catalysts, high reaction temperature, etc., and achieve the effects of high yield and purity, low production cost and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 58 g (1.00 mol) of potassium fluoride and 300 mL of N,N-dimethylformamide (DMF) into a 500 ml four-necked flask equipped with mechanical stirring, a thermometer, and a vacuum distillation device, and heat up to about 50 °C , adjust the vacuum degree to -0.090MPa, a small amount of DMF can be distilled during this period, continue to heat up to about 70-80 ℃, distill about 80-100 mL of DMF to remove the moisture in the raw materials and solvent as much as possible, then remove the vacuum, and cool down to 60 -65°C, add 3.2 g (0.01 mol) tetrabutylammonium bromide and 32.2 g (0.19 mol) 4,6-dichloro-5-fluoropyrimidine, and stir the reaction at this temperature for 5 h. After the complete reaction of the raw materials was detected by gas chromatography, the reaction device was changed to an atmospheric distillation device, and the fraction with a gas phase temperature of 85-86°C was collected to obtain 21.5 g of colorless and transparent liquid 4,5,6-trifluoropyrimidine, ...
Embodiment 2
[0026] Add 58 g (1.00 mol) of potassium fluoride and 300 mL of dimethyl sulfoxide (DMSO) into a 500 ml four-necked flask equipped with mechanical stirring, a thermometer, and a vacuum distillation device, raise the temperature to about 90 °C, and adjust the vacuum degree To -0.095MPa, a small amount of DMSO can be distilled during this period, continue to heat up to about 100-110 ℃, distill about 80-100 mL DMSO to remove the moisture in the raw material and solvent as much as possible, then remove the vacuum, cool down to 55-60 ℃, Add 1.6 g (0.005 mol) tetrabutylammonium bromide and 32.2 g (0.19 mol) 4,6-dichloro-5-fluoropyrimidine, and stir the reaction at this temperature for 7 h. After the reaction of the raw materials was detected by gas chromatography, the reaction device was changed to an atmospheric distillation device, and the fraction with a gas phase temperature of 85-86 ° C was collected to obtain 21.9 g of 4,5,6-trifluoropyrimidine, a colorless transparent liquid, w...
Embodiment 3
[0028] Add 174g (3.00 mol) of potassium fluoride and 600 mL of sulfolane into a 1000 ml four-necked flask equipped with mechanical stirring, a thermometer, and a vacuum distillation device, raise the temperature to about 130°C, and adjust the vacuum to -0.098MPa. Produce a small amount of sulfolane, continue to heat up to about 140-150 °C, distill about 80-100 mL of sulfolane to remove the moisture in the raw material and solvent as much as possible, then remove the vacuum, cool down to 60-65 °C, add 3.2 g (0.01 mol) tetra Butylammonium bromide and 64.5g (0.39mol) 4,6-dichloro-5-fluoropyrimidine were stirred at this temperature for 5h. After the complete reaction of the raw materials was detected by gas chromatography, the reaction device was changed to an atmospheric distillation device, and the fraction with a gas phase temperature of 85-86 ° C was collected to obtain 47.5 g of colorless and transparent liquid 4,5,6-trifluoropyrimidine, which was detected by GC with a purity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com