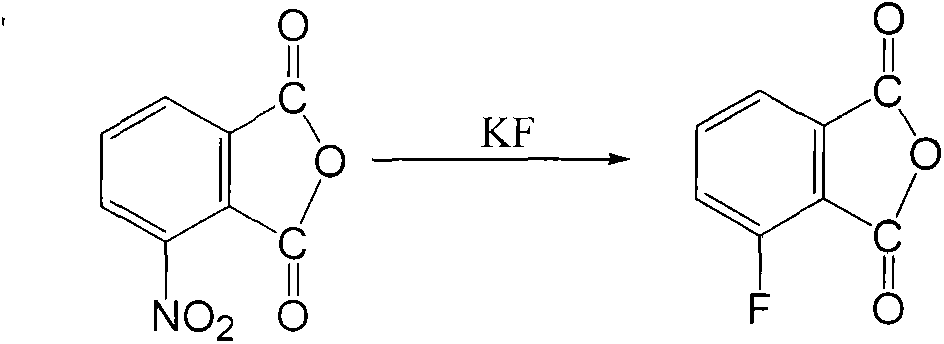
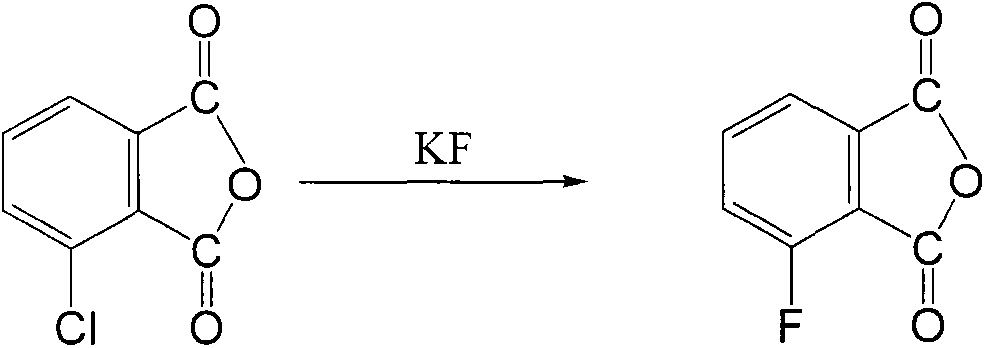Preparation method of 3-fluorophthalic anhydride
A kind of technology of fluorophthalic anhydride and chlorophthalic anhydride, applied in the field of preparation of 3-fluorophthalic anhydride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 500mL four-neck flask, add 100g of 3-chlorophthalic anhydride and 100mL of hydrogen fluoride pyridine complex, protect it with nitrogen, stir the reaction at 100-160°C, monitor the reaction progress by GC, when the conversion rate of 3-chlorophthalic anhydride reaches 98.0% When above, stop the reaction; pour the reaction liquid into 2000mL ice water, stir and mix thoroughly, precipitate the solid, filter, rinse the solid with ice water, and dry in vacuo to obtain 82.6g of crude product of 3-fluorophthalic anhydride; 160 mL of n-heptane was fully washed, filtered, and vacuum-dried to obtain 76.4 g of pure 3-fluorophthalic anhydride, with a yield of 84.0% and a melting point of 158-160°C.
Embodiment 2
[0031] In a 500mL four-neck flask, add 100g of 3-chlorophthalic anhydride and 100mL of hydrogen fluoride pyridine complex, protect it with nitrogen, stir the reaction at 120-140°C, monitor the reaction progress by GC, when the conversion rate of 3-chlorophthalic anhydride reaches 98.0 % above, terminate the reaction; pour the reaction solution into 4000mL ice water, stir and mix thoroughly, precipitate the solid, filter, rinse the solid with ice water, and dry in vacuo to obtain 81.8g of crude 3-fluorophthalic anhydride; Wash thoroughly with 200mL of n-pentane, filter, and vacuum-dry to obtain 75.8g of pure 3-fluorophthalic anhydride, with a yield of 83.3% and a melting point of 159-161°C.
Embodiment 3
[0033] In a 500mL four-neck flask, add 100g of 3-chlorophthalic anhydride and 100mL of hydrogen fluoride triethylamine complex, protect it with nitrogen gas, stir the reaction at 100-180°C, monitor the reaction progress by GC, when the conversion rate of 3-chlorophthalic anhydride When it reaches above 98.0%, the reaction is terminated; the reaction solution is poured into 2000mL ice water, fully stirred and mixed, the solid is precipitated, filtered, the solid is rinsed with ice water, and vacuum-dried to obtain 79.8g of crude 3-fluorophthalic anhydride; 160 mL of toluene and 200 mL of n-pentane were fully washed, filtered, and vacuum-dried to obtain 71.1 g of pure 3-fluorophthalic anhydride, the yield was 78.2%, and the temperature was 159-161 ° C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com