Rhodanine derivative and preparation method thereof
A technology of derivatives and anti-tumor drugs, applied in the field of rhodanine derivatives and its preparation, can solve the problems of cytotoxicity, short half-life of sodium hydrosulfide, DNA damage of human lung fibroblasts, etc., and achieve good inhibitory effect and strong activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
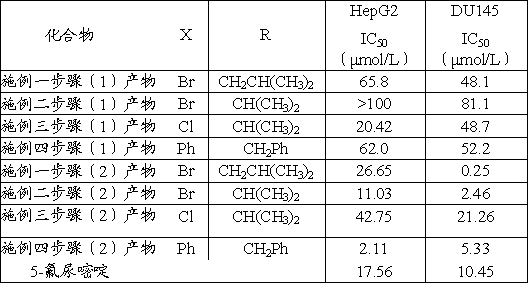
[0019] (1) Add 0.494g, 2.0mmol of 2-[-4-oxo-2-thio-thiazolidin-3-yl]-4-methylpentanoic acid, 0.368g, 2.0mmol of p-bromobenzaldehyde and Add 0.313g and 4.1mmol of ammonium acetate to 20mL of toluene, heat and reflux for 1h, evaporate the solvent under reduced pressure, and perform column chromatography with eluent petroleum ether and ethyl acetate at a volume ratio of 4:1 to obtain a light yellow solid, the product The rate is 75.9%, and the melting point is 148.5~152.0°C. The hydrogen spectrum of the product Z-2-[5-(4-bromobenzylidene)-4-oxo-2-thio-thiazolidin-3-yl]-4-methylpentanoic acid 1 H NMR (400MHz, CDCl 3 ) δ: 0.94(d, 3H, J=6.6Hz, CH 3 ), 0.98(d, 3H, J=6.5Hz, CH 3 ), 1.48~1.61(m, 1H, CH), 2.08~ 2.16(m, 1H, CH 2 ), 2.25~2.32(m, 1H, CH 2 ), 5.79~5.80(m, 1H, CH), 7.35(d, 2H, J=8.4Hz, ArH), 7.62(d, 2H, J=8.0Hz,ArH), 7.63(s, 1H, =CH) .
[0020] (2) 495.576mg, 1.2mmol of Z-2-[5-(4-bromobenzylidene)-4-oxo-2-thio-thiazolidin-3-yl]-4-methylpentanoic acid , 299mg, 1.5mmol...
Embodiment 2
[0023] (1) Using p-bromobenzaldehyde and 2-(4-oxo-2-thio-thiazolidin-3-yl)-3-methylbutyric acid as raw materials, the preparation process is the same as step (1) of Example 1, A light yellow solid was obtained with a yield of 78.4% and a melting point of 157.9~160.1°C. The hydrogen spectrum of the product Z-2-[5-(4-bromobenzylidene)-4-oxo-2-thio-thiazolidin-3-yl]-3-methylbutanoic acid 1 H NMR (400MHz, CDCl 3)δ: 0.83(d, 3H, J=6.8Hz, CH 3 ), 1.27(d, 3H, J=6.3Hz, CH 3 ), 2.83~2.92(m, 1H, CH), 5.37(d, 1H, J=9.1Hz, CH), 7.35(d, 2H, J=8.4Hz, ArH), 7.62(d, 2H, J=8.4 Hz, ArH), 7.64(s, 1H, =CH).
[0024] (2) With Z-2-[5-(4-bromobenzylidene)-4-oxo-2-thio-thiazolidin-3-yl]-3-methylbutanoic acid and 5-p-hydroxybenzene Base-1,2-dithiole-3-thione was used as the raw material, and the preparation process was the same as step (2) of Example 1 to obtain a red solid with a yield of 73.4% and a melting point of 159.5~160.7°C. Product Z-2-[5-(4-bromobenzylidene)-4-oxo-2-thio-thiazolidin-3-y...
Embodiment 3
[0027] Using p-chlorobenzaldehyde and 2-(4-oxo-2-thio-thiazolidin-3-yl)-3-methylbutanoic acid as raw materials, the preparation process is the same as step (1) of Example 1 to obtain light Yellow solid, yield 80.3%, melting point 152.0~154.3°C. The hydrogen spectrum of the product Z-2-[5-(4-chlorobenzylidene)-4-oxo-2-thio-thiazolidin-3-yl]-3-methylbutanoic acid 1 H NMR (400MHz, CDCl 3 )δ: 0.87(d, 3H, J=6.6Hz, CH 3 ), 1.26(d, 3H, J=6.9Hz, CH 3 ), 2.84~ 2.93(m, 1H, CH), 5.37(d, 1H, J=9.0Hz, CH), 7.41~7.47(m, 4H, ArH), 7.66(s, 1H, =CH).
[0028] With Z-2-[5-(4-chlorobenzylidene)-4-oxo-2-thio-thiazolidin-3-yl]-4-methylbutanoic acid and 5-p-hydroxyphenyl-1 , 2-dithiole-3-thione was used as the raw material, and the preparation process was the same as step (2) of Example 1 to obtain a red solid with a yield of 70.1% and a melting point of 164.3~165.2°C. Product Z-2-[5-(4-chlorobenzylidene)-4-oxo-2-thioketothiazolidin-3-yl]-4-methylbutanoic acid 4-(3H-1,2-di Thiol-3-thiol-5-yl)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com