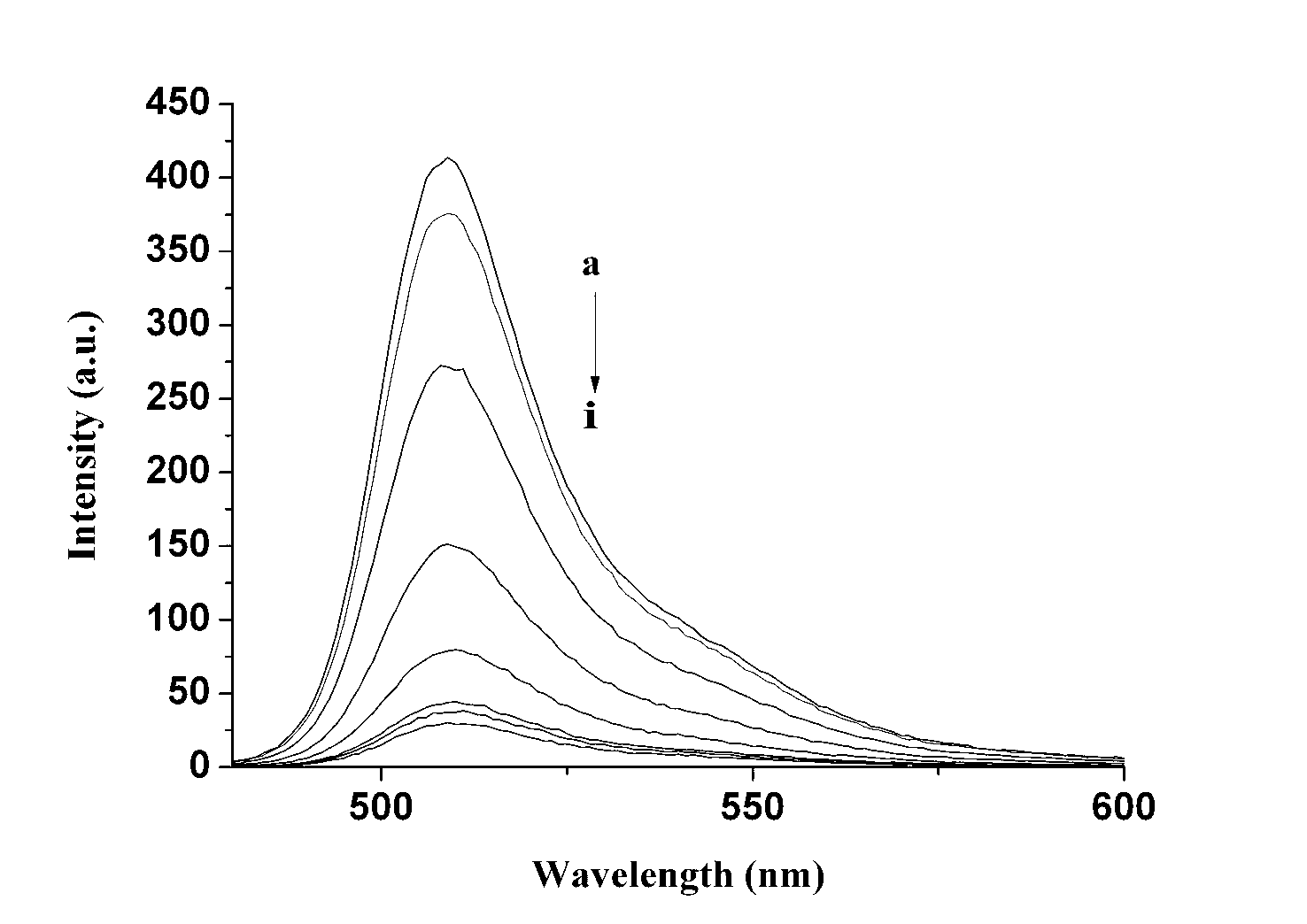
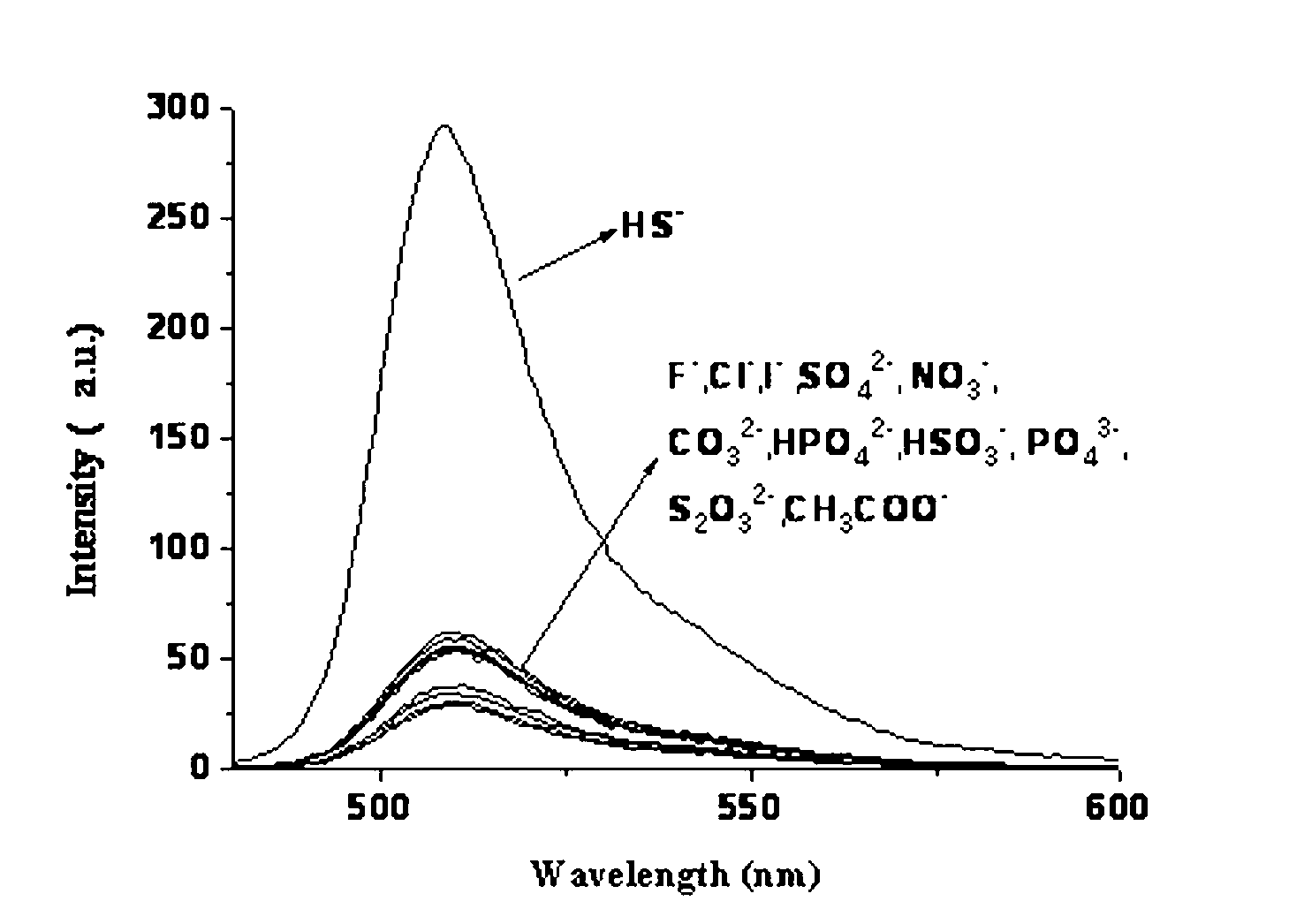
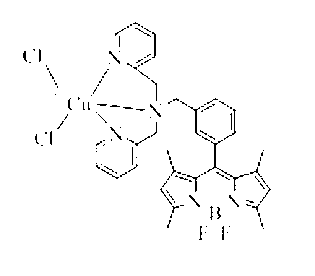
Sulfhydryl group ion fuorescence probe and synthesis method thereof
A fluorescent probe and hydrogen sulfide technology, applied in the field of analytical chemistry, can solve the problems of processing a large number of biological tissue samples, expensive testing instruments, etc., and achieve the effect of low toxicity and rapid response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]The compound DOBIPY-DPA (53.5mg, 0.1mmol) was mixed with CuCl 2 (13.5mg, 0.1mmol) were mixed at a molar ratio of 1:1, dissolved in methanol solution (30mL), and reacted at room temperature for 30min to obtain the compound dichloride [8-bis(2-pyridylmethyl)amine-3-benzyl Base-4,4-difluoro-1,3,5,7-tetramethyl-4-boron-3a,4a-dipyrrole]copper (abbreviated as DOBIPY-DPA-Cu). Yield 96%. ES-MS (CH 3 OH): m / z 633.42(100)[(DOBIPT-DPA-Cu)-Cl - ] + .
Embodiment 2
[0031] Compound DOBIPY-DPA (53.5mg, 0.1mmol) and CuCl 2 (6.8mg, 0.05mmol) were mixed at a molar ratio of 2:1, dissolved in methanol solution (60mL), and reacted at room temperature for 60min to obtain the compound dichloride [8-bis(2-pyridylmethyl)amine-3-benzyl Base-4,4-difluoro-1,3,5,7-tetramethyl-4-boron-3a,4a-dipyrrole]copper (abbreviated as DOBIPY-DPA-Cu). Yield, 60%.
Embodiment 3
[0033] Compound DOBIPY-DPA (53.5mg, 0.1mmol) and CuCl 2 (27mg, 0.2mmol) were mixed at a molar ratio of 1:2, dissolved in methanol solution (60mL), and reacted at room temperature for 5min to obtain the compound dichloride [8-bis(2-pyridylmethyl)amine-3-benzyl -4,4-difluoro-1,3,5,7-tetramethyl-4-boron-3a,4a-dipyrrole]copper (abbreviated as DOBIPY-DPA-Cu). Yield, 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com