Reference substance for measuring relative molecular weight and molecular weight distribution of heparin and salt thereof
A technology of molecular weight distribution and molecular weight, which is applied in the field of reference substances used to determine the relative molecular weight and molecular weight distribution of heparin and its salts, can solve problems such as inaccurate measurement results, inability to cover heparin molecules in the molecular weight range, and inaccurate relative molecular weight, etc., to achieve The determination method is simple, the prospect of popularization and application is good, and the effect of high practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation process of heparin molecular weight reference substance
[0037] Materials and reagents: 10 batches of heparin sodium samples were selected, all of which were domestic heparin sodium injection grade raw materials; sodium nitrate, sodium azide, domestic analytical grade; triple distilled water.
[0038] Preparation of mobile phase: Accurately weigh 34g of sodium nitrate and 0.4g of sodium azide, dissolve them in 2L of triple distilled water. Suction filter twice with a double-layer 0.22 μm aqueous microporous membrane.
[0039] Preparation of sample solution: Accurately weigh 6 mg of heparin sodium raw material drug sample and dissolve in 4 mL of mobile phase. Double-layer 0.22μm aqueous microporous membrane filtration once.
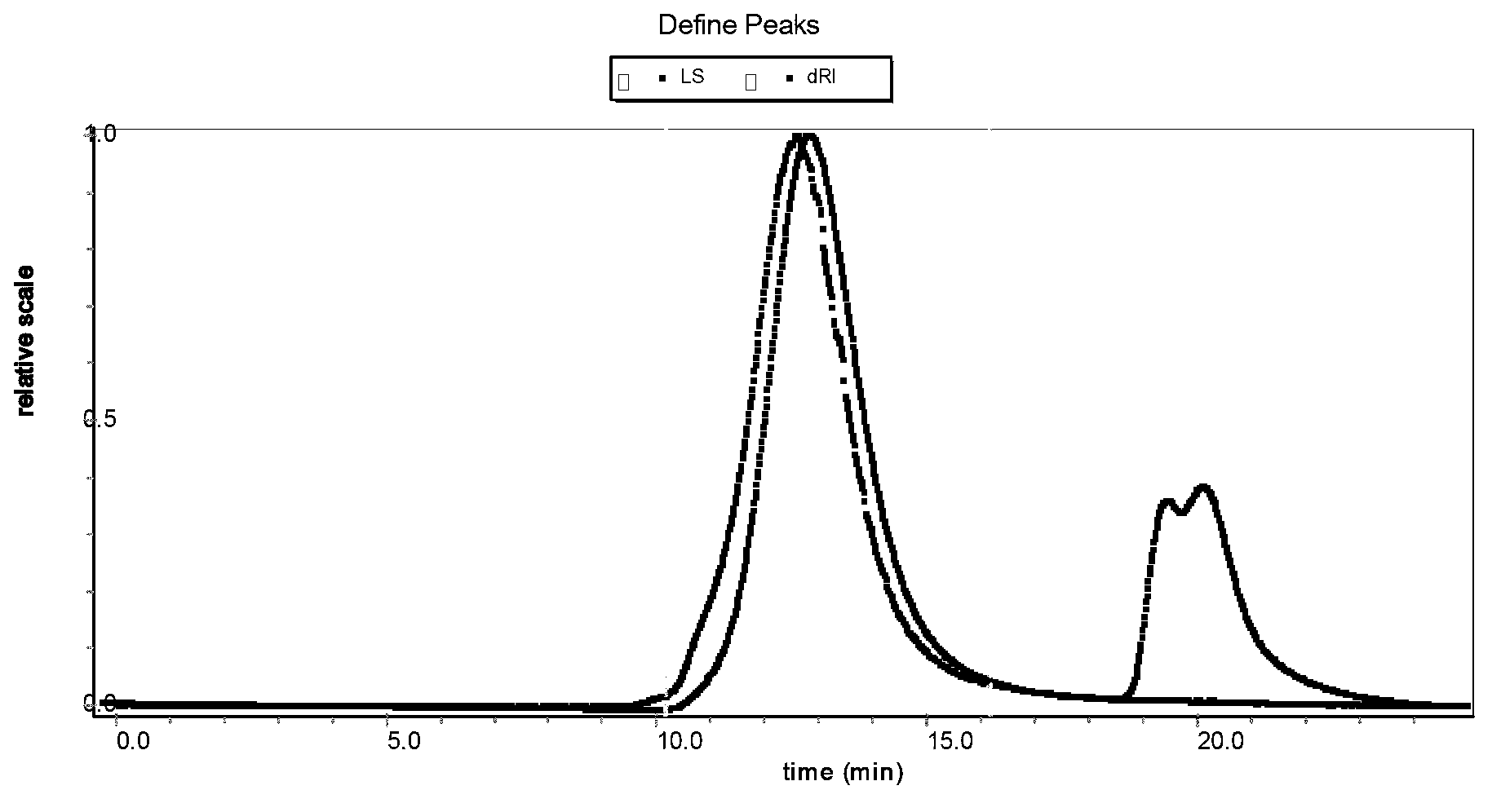
[0040]Chromatography system: Waters515HPLC pump, Wyatt Optilab rEX parallax refractive index detector, Wyatt DAWN HELEOS II multi-angle laser scattering instrument.
[0041] The molecular weight and molecular weight distrib...
Embodiment 2
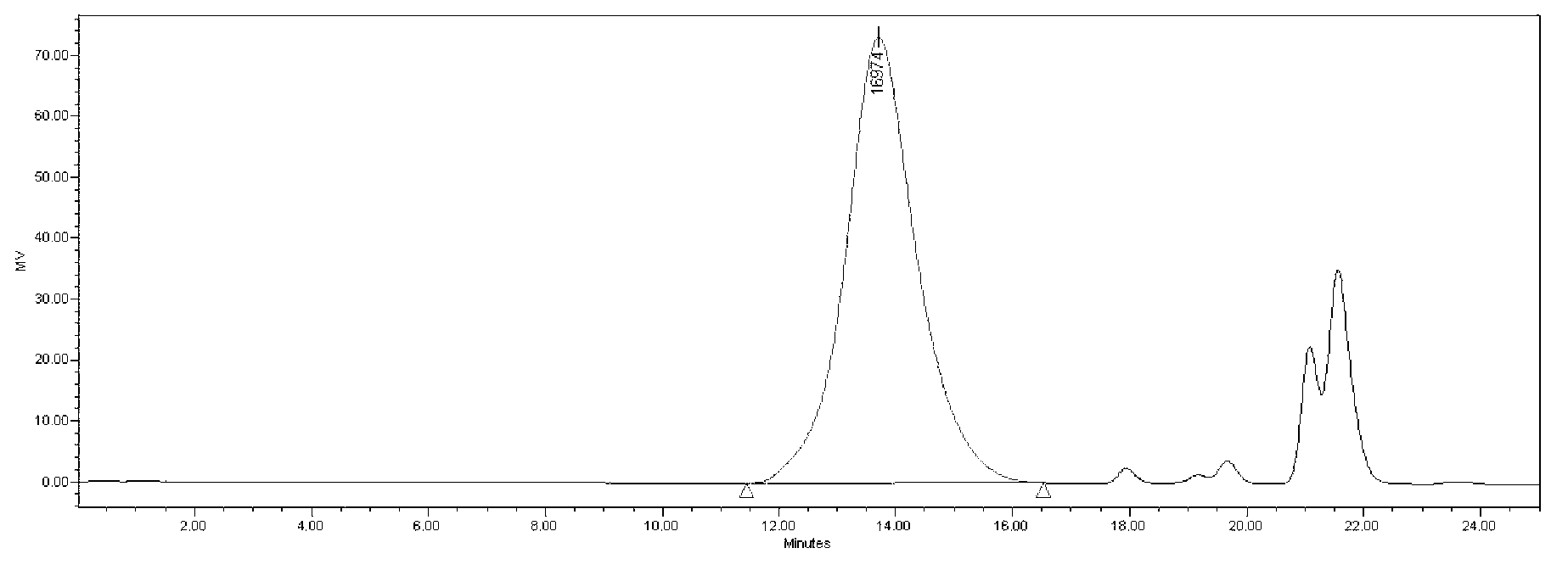
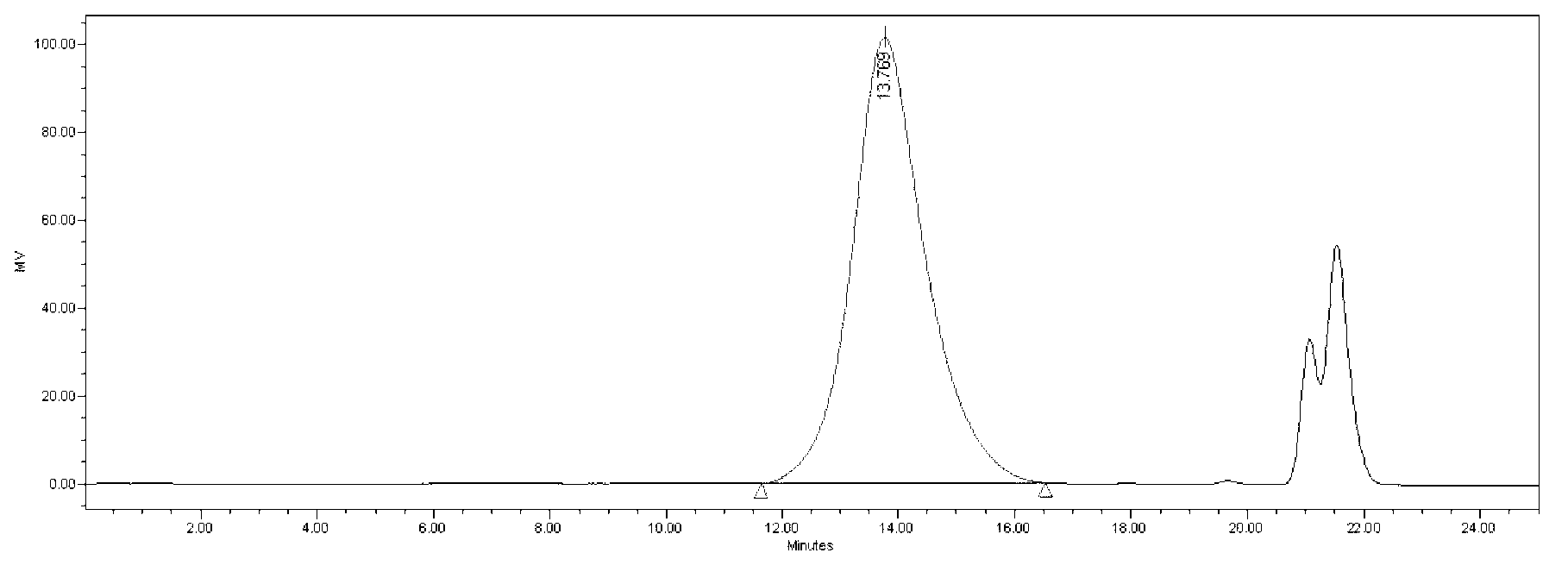
[0055] Example 2 Determination of relative molecular weight and molecular weight distribution of heparin sodium test product by using heparin molecular weight reference substance
[0056] (1) HPSEC analysis of heparin molecular weight reference substance and heparin test substance
[0057] Materials and reagents: 10 batches of heparin sodium samples were selected, all of which were domestic heparin sodium injection grade raw materials; sodium nitrate, sodium azide, domestic analytical grade; triple distilled water.
[0058] Chromatographic system: Waters2695, 2414.
[0059] The chromatographic conditions are as shown in Table 7:
[0060] Table 7
[0061]
[0062] Preparation of mobile phase: Accurately weigh 34g of sodium nitrate and 0.4g of sodium azide, dissolve them in 2L of triple distilled water. Suction filter twice with a double-layer 0.22 μm aqueous microporous membrane.
[0063] Preparation of the reference substance and the test solution: 10 mg of the samples ...
Embodiment 3
[0076] Embodiment 3 Precision experiment
[0077] Take 5 batches of heparin sodium samples, all of which are domestic injection-grade heparin sodium raw materials. Using the experimental conditions and methods in Example 2, each batch of samples was continuously injected 5 times to determine their relative molecular weight. The results are shown in Tables 11-20. Show:
[0078] Table 11 Batch 1 Molecular Weight Determination Precision Experimental Results
[0079]
[0080]
[0081] Table 12 Batch 1 molecular weight distribution precision test results
[0082]
[0083] Table 13 Batch 2 Molecular Weight Determination Precision Experimental Results
[0084]
[0085] Table 14 Batch 2 molecular weight distribution precision test results
[0086]
[0087] Table 15 Batch 3 Molecular Weight Determination Precision Experimental Results
[0088]
[0089] Table 16 Batch 3 molecular weight distribution precision test results
[0090]
[0091]
[0092] Table 17 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com