High performance liquid chromatography method and application thereof
A technology of high-performance liquid chromatography and analysis method, which is applied in the field of high-performance liquid chromatography analysis method and its microbial conversion of phytosterols to produce androstenedione, which can solve problems such as difficult analysis, many operation steps, and long analysis time, and achieve Effects of shortening analysis time, changing operation steps, and eliminating interference factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1: the selection of chromatographic conditions
[0096] 1.1 Selection of mobile phase
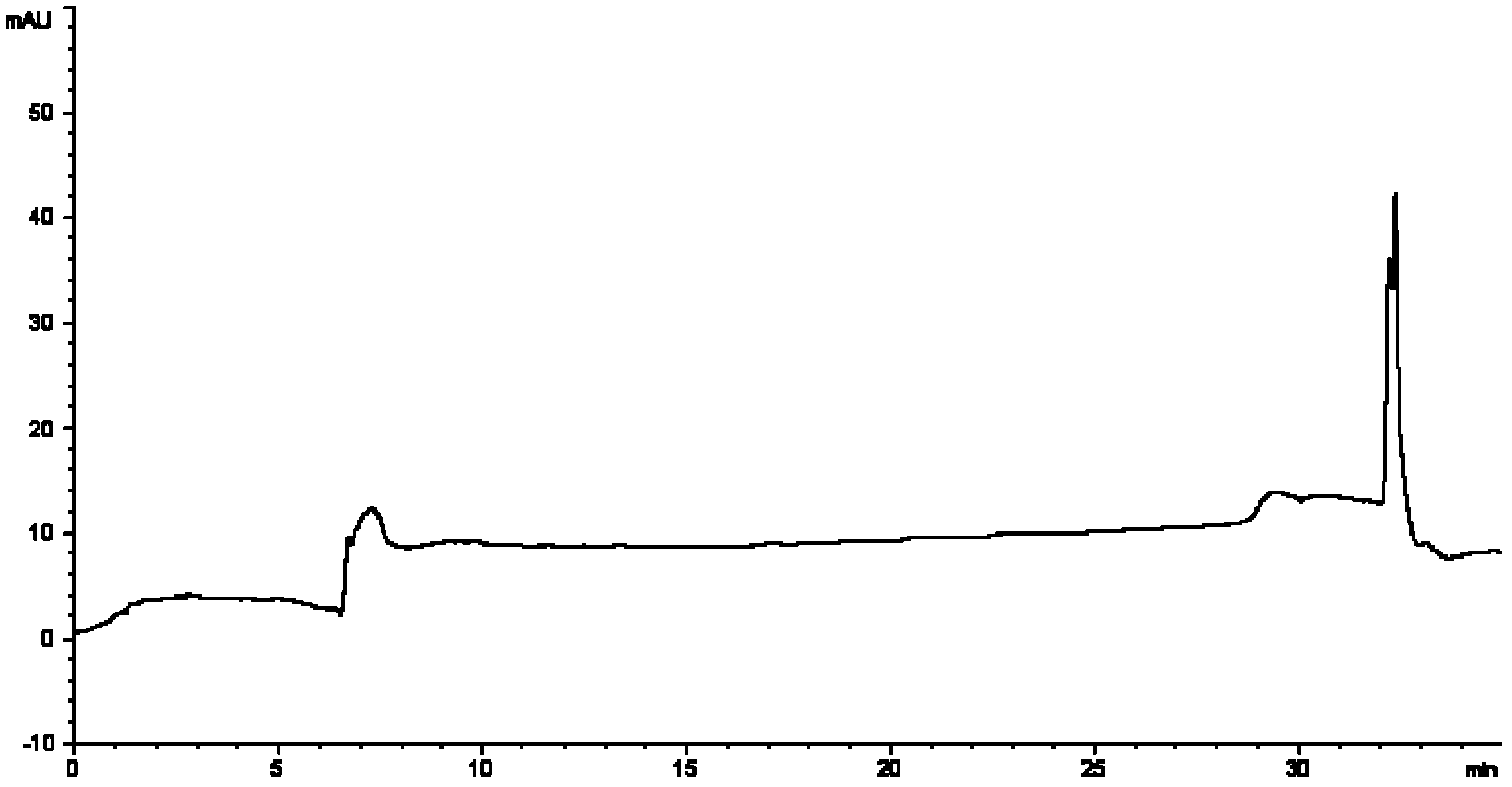
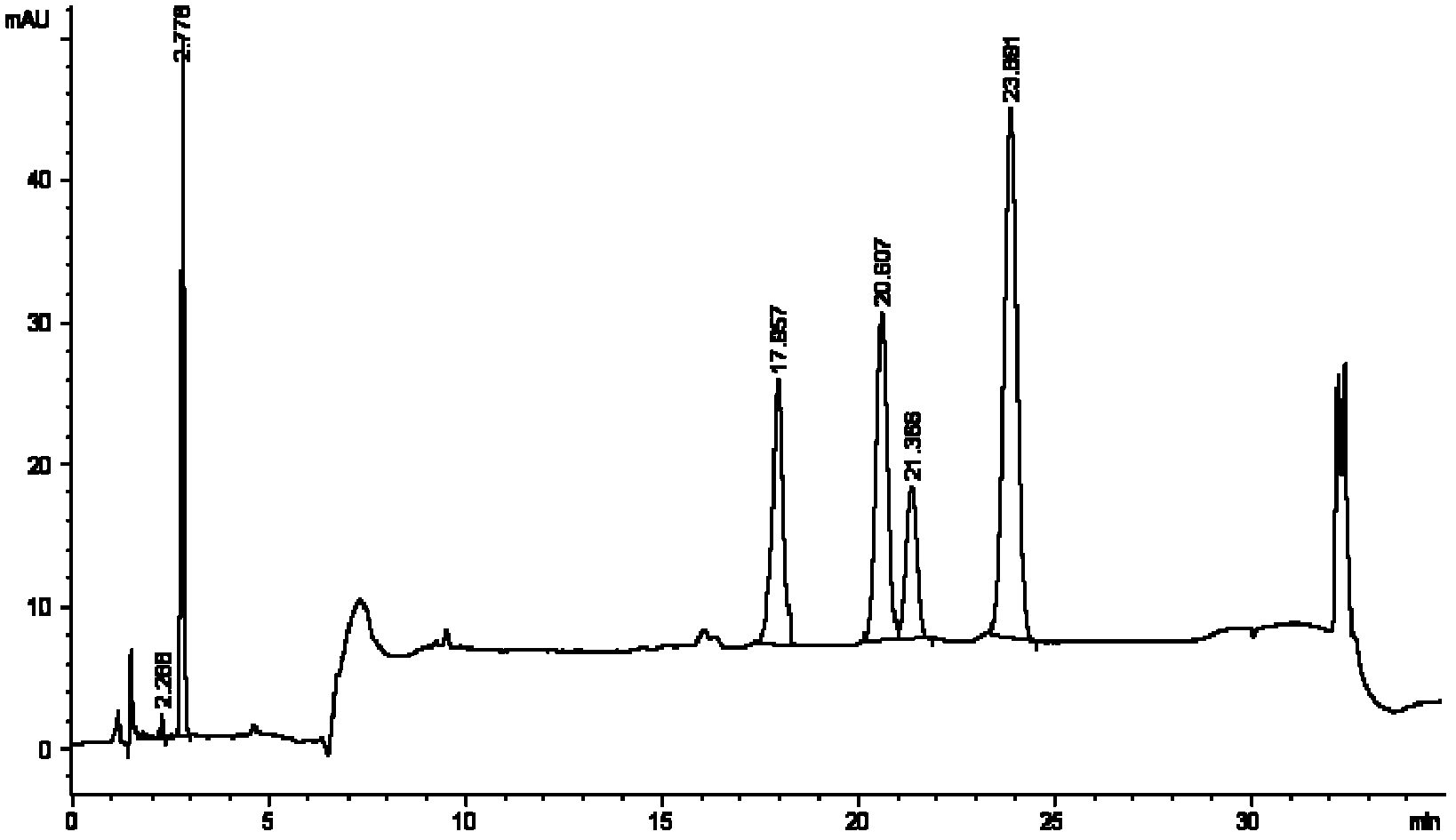
[0097] The reason why the present invention selects acetonitrile and water as the mobile phase is for the consideration of the following factors: eliminate the interference factors of mobile relative detection during measurement to improve reliability and accuracy; improve the separation between each peak; and use gradient washing To achieve effective separation of androstenedione from impurities before and after, and effective separation of brassicasterol, campesterol, stigmasterol, and β-sitosterol; see blank figure 1 , mixed standard figure 2 (The peak order is androstenedione (AD), brassicasterol, campesterol, stigmasterol, β-sitosterol).
[0098] 1.2 Selection of detection wavelength
[0099] The sample is dissolved in acetonitrile, and the DAD detector is used for continuous scanning at 190-400nm. It can be seen that androstenedione (AD) has a maximum absorption ...
Embodiment 2
[0111] Preparation of Standard Curve
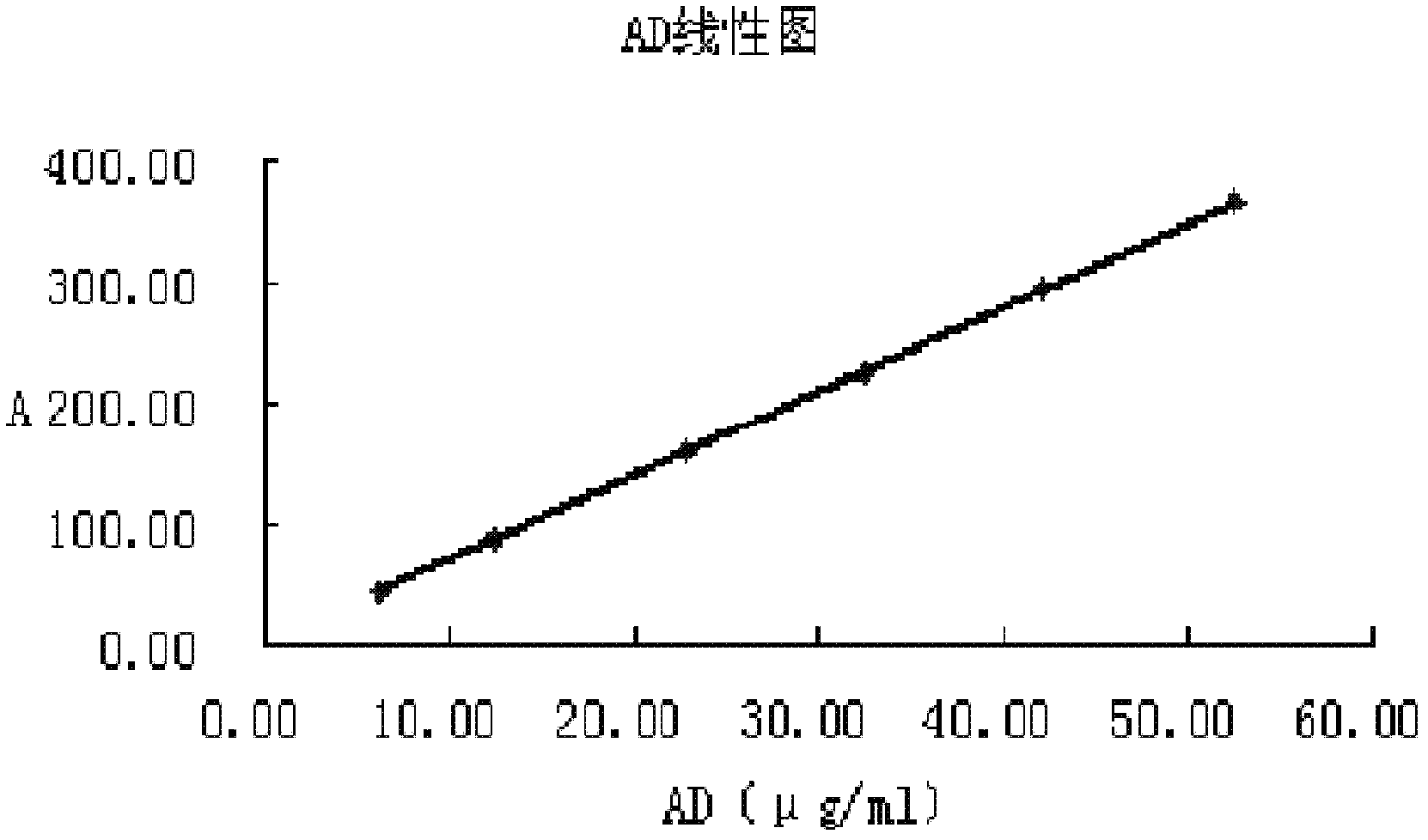
[0112] The concentrations of androstenedione (AD), brassicasterol, campesterol, stigmasterol and β-sitosterol are about 6.22-52.65μg / ml, 28.0-405.0μg / ml, 27.9-402.6μg / ml, 28.22- 407.2μg / ml and 27.93-402.9μg / ml, inject 10μl, plot the peak area A against the concentration μg / ml, see image 3 , Figure 4 , Figure 5 , Figure 6 , Figure 7 , the regression equation is:
[0113] Androstenedione (AD) y=6.9463x+0.12r=0.99998;
[0114] Brassicasterol y=1.8587x+1.1906r=0.99986;
[0115] Campesterol y=2.4101x+0.7561r=0.99993;
[0116] Stigmasterol y=1.0758x+1.9945r=0.99995;
[0117] β-sitosterol y=1.0767x+1.9858r=0.99993;
Embodiment 3
[0119] Determination of standard repeatability
[0120] Mixed standard solution:
[0121]Accurately pipette an appropriate amount of each standard stock solution, dilute to volume with acetonitrile in the same 25ml volumetric flask, shake well, and filter with an organic 0.22 μm filter membrane to obtain the mixed standard solution; make about androstenedione (AD) (32.0μg / ml), brassicasterol (204.0μg / ml), campesterol (203.0μg / ml), stigmasterol (205.0μg / ml), β-sitosterol (203.0μg / ml), injection 10μl, Six needles were injected continuously, and the RSD% calculated by the peak area is shown in Table 4.
[0122] Table 4 Repeatability test result table
[0123]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com