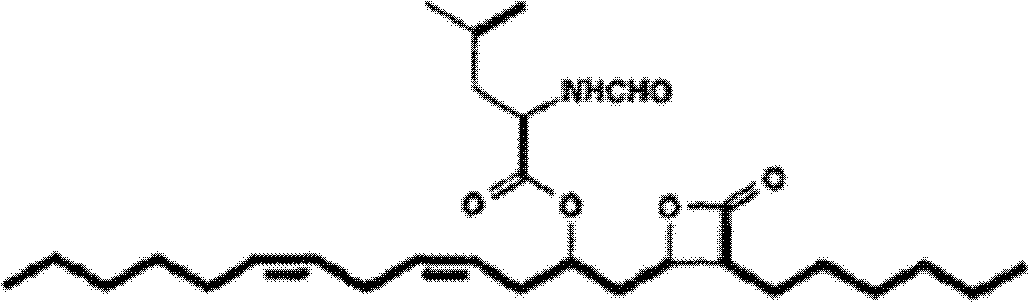
Method for purifying lipstatin
A purification method and the technology of Lipus, which are applied in the field of purification of Lipistatin, can solve the problems of increasing production safety risks, increasing the recovery workload, increasing hidden costs, etc., so as to reduce the number of high-temperature burning and regeneration, and improve the use of Longevity and the effect of improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0041] A method for purifying liprestatin, comprising:
[0042] 1) providing a solid mixture containing riprestatin;
[0043] 2) extracting the solid mixture containing riprestatin obtained in step 1) with a low-polarity or non-polar organic solvent as an extractant to obtain an extract containing riprestatin;
[0044] 3) Adsorbing the liprestatin-containing extract obtained in step 2) with molecular sieves, and eluting with the first eluent, thereby obtaining the first eluate containing liprestatin; and
[0045] 4) The first eluate containing riprestatin obtained in step 3) is further purified by chromatography.
[0046] Wherein, the solid mixture containing riprestatin in the above-mentioned step 1) is a solid mixture containing liprestatin obtained from fermentation broth or other means, and the content of riprestatin in the solid mixture is not particularly limited . Preferably, the solid mixture containing liprestatin obtained by drying the fermented liquid of Streptom...
Embodiment 1
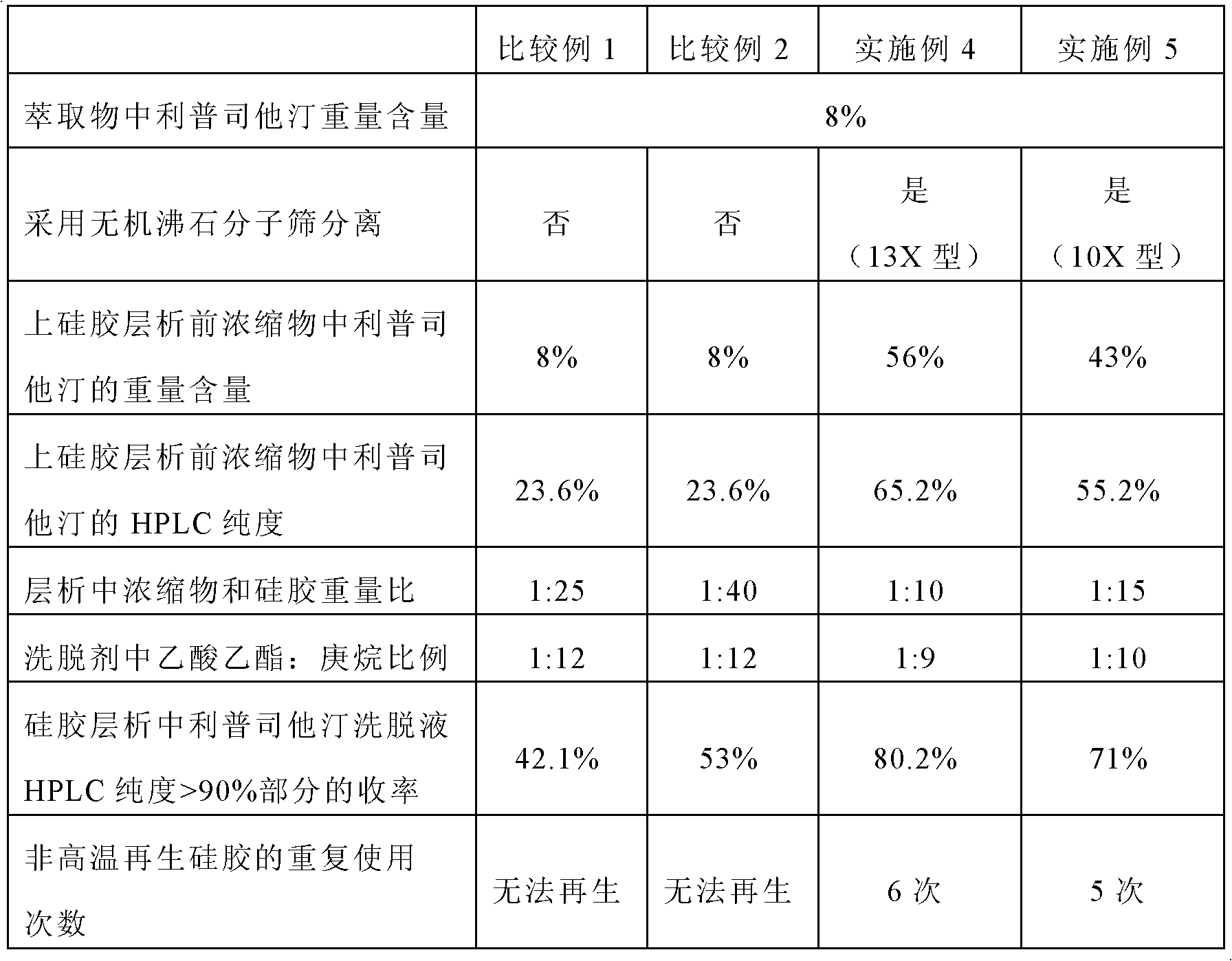
[0077] 1000 g of riprestatin fermentation broth was spray-dried and extracted twice with heptane, the extraction temperature was 30°C, and the amount of heptane used for the first extraction and the second extraction was 5 L and 3 L. The liprestatin in the heptane extraction mixture was detected by HPLC, and its purity was measured to be 20.59%. A small amount of the heptane extraction mixture was concentrated under reduced pressure and vacuum at 40° C. to obtain a concentrated paste, which was measured to have a weight content of 8%; the amount of riprestatin product contained in the remaining extraction mixture was 6.5 g.
[0078] The diameter-to-height ratio of the molecular sieve is 1:7. The 13X molecular sieve is used for molecular screening. Weigh 50 g of the 13X molecular sieve, and soak the molecular sieve in heptane for more than 8 hours before use. The heptane extraction mixture passed through the molecular sieve column at 25°C and a flow rate of 4BV / h. After complet...
Embodiment 2
[0080] 1000 g of riprestatin fermentation broth was sprayed to dry residue and extracted twice with petroleum ether, the extraction temperature was 30°C, and the amount of petroleum ether used for the first extraction and the second extraction was 5 L and 3 L. The purity of riprestatin in the petroleum ether extraction mixture detected by HPLC was 22.7%. A small amount of petroleum ether extraction mixture was concentrated under reduced pressure and vacuum at 40° C. to obtain a concentrated paste with a measured weight content of 7.6%; the content of riprestatin in the remaining extraction mixture was 6 g.
[0081] The diameter-to-height ratio of the molecular sieve is 1:8, and the molecular sieve is a 10X molecular sieve. Weigh 63g of the 10X molecular sieve, and soak the molecular sieve in petroleum ether for more than 8 hours before use. The petroleum ether extraction mixture passed through the molecular sieve column at 25°C and a flow rate of 3BV / h. After completion, conti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com