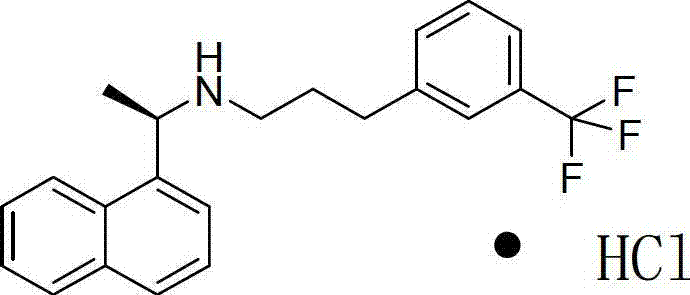
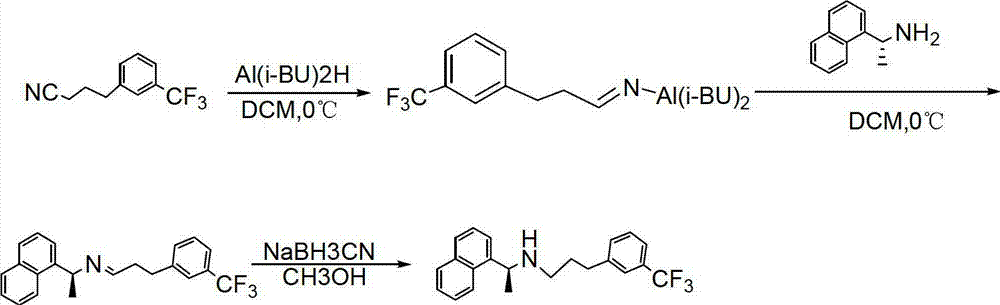
Preparation method of high-purity cinacalcet hydrochloride
A technology of cinacalcet hydrochloride and vacuum degree, which is applied in the preparation of amino-substituted functional groups, purification/separation of amino compounds, organic chemistry, etc. Low cost, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of m-trifluoromethylphenylpropyl bromide crude product
[0042] Under stirring at room temperature, 3-trifluoromethylphenylpropanol (150g, 734.6mmol) was added to the mixed solution of water: concentrated sulfuric acid (5:7, 360ml), and lithium bromide monohydrate (210g, 2002.67mmol) was slowly added, Heat to reflux at 90°C for about 4 hours, TCL detection (PE: EA = 20: 1), after the reaction is complete, cool down to room temperature, filter out the solid with suction (210ml of ethyl acetate rinse), separate the obtained liquid, wash with water (300ml× 2), washed with saturated sodium bicarbonate (300ml×2), washed with water (300ml×1), dried over anhydrous sodium sulfate, and spin-dried in the organic phase to obtain 225g of crude product.
[0043] The crude yield of this step reaction is 95%-100%.
Embodiment 2
[0044] Embodiment 2: the preparation of m-trifluoromethyl phenylpropyl bromide fine work
[0045] 225g of m-trifluoromethylphenylpropyl bromide crude product was subjected to oil pump rectification, vacuum: 133-266 Pa, external temperature 85-90 degrees, headspace 58-60 degrees, collected fractions, and obtained 466g of colorless oily product, collected Rate: 87%.
Embodiment 3
[0046] Embodiment 3: the preparation of cinacalcet hydrochloride crude product
[0047] Add 275g of (R)-α-naphthylethylamine and 1580g of acetonitrile into a 5L reactor, add 450g of m-trifluoromethylphenylpropyl bromide, 222kg of anhydrous potassium carbonate under stirring, heat and reflux at 81°C for 20h-22h, TLC Detecting the reaction, m-trifluoromethylphenylpropyl bromide is basically completely consumed, and the reaction is terminated, and the reaction drops to 25-30 degrees.
[0048] Post-processing: filter to remove the solid in the reaction solution, rinse with 158g of acetonitrile, add the filtrate to 8.2kg of water, add 317g of concentrated hydrochloric acid dropwise, adjust the pH<2, stir and precipitate the solid, continue to stir for 2 hours, and filter with suction to get a earthy color Solid, the obtained crude product was placed in a 10L reaction kettle, 8kg of purified water was added, stirred and washed at room temperature for 3 hours, and the earth-colored s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com