Fluorine-cation-containing WPU (waterborne polyurethane) and preparation method thereof
A water-based polyurethane and cationic technology, applied in the field of chemical materials, can solve the problems of high fluorine content and high production cost, and achieve the effects of stable performance, improved solvent resistance and mechanical properties, and controllable raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
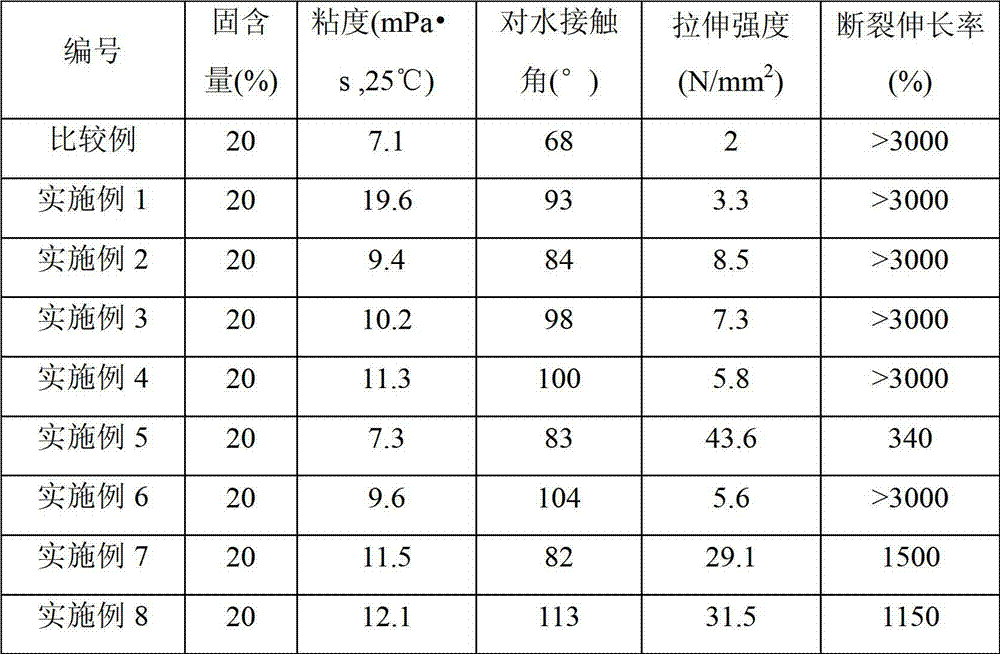
Examples
Embodiment 1
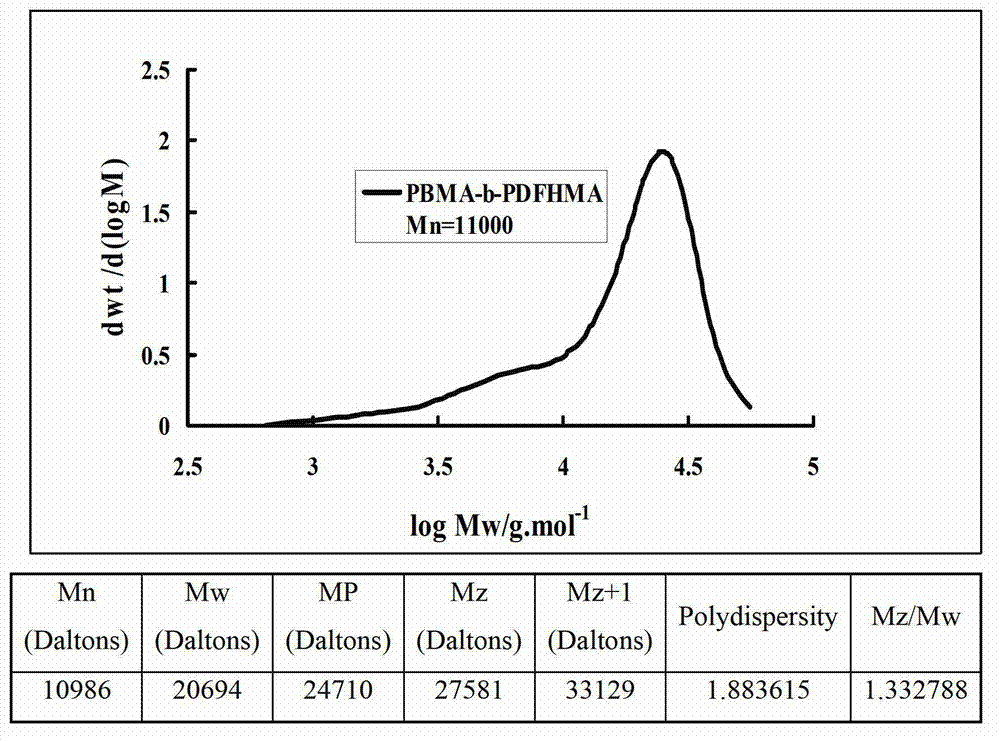
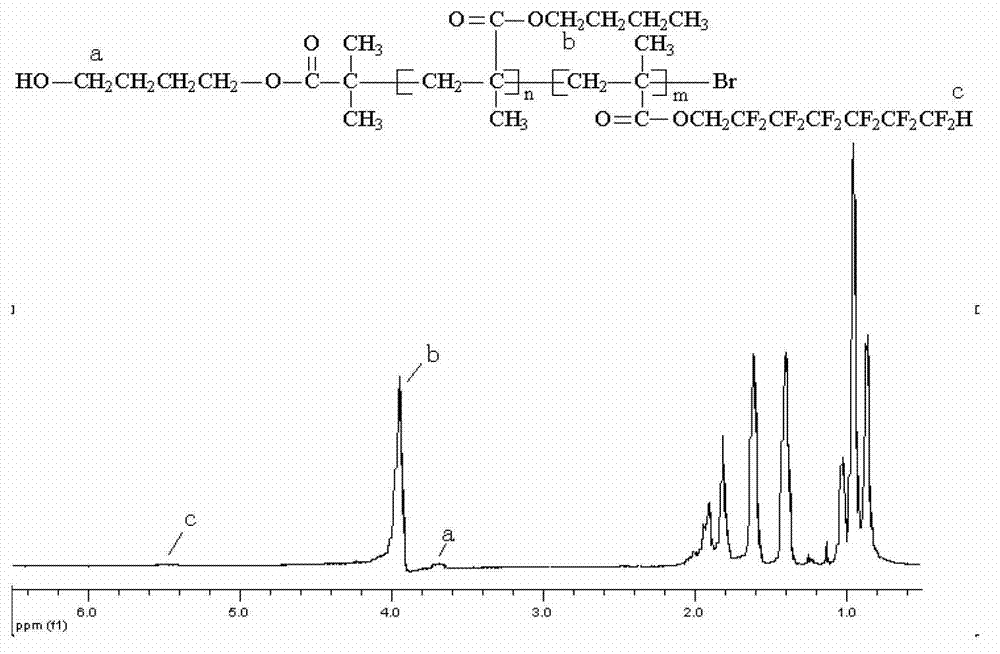
[0037] (1) Preparation of fluorine-containing block acrylate copolymer: preparation of block copolymer with molecular weight of 10,000 and block mass ratio of 9:1 terminal hydroxyl butyl methacrylate and dodecafluoroheptyl methacrylate
[0038] In a 100mL four-necked flask, add 0.498g (0.002mol) initiator α-bromoisobutyric acid (4-hydroxybutylene glycol ester), 18g (0.127mol) of butyl methacrylate monomer, 0.183g (0.00127 mol) catalyst CuBr, 0.439g (0.00254mol) ligand PMDETA, 7.2g solvent toluene, mix well. The system was evacuated and filled with nitrogen, and stirred and reacted in an oil bath at 60°C. After 8 hours, add 2 g (0.005 mol) dodecafluoroheptyl methacrylate, 0.0072 g (0.00005 mol) CuBr, 0.0173 g (0.0001 mol) ligand PMDETA to the flask. The stirring reaction was continued under the bath for 12 hours. After the reaction was completed, the catalyst and solvent were removed to obtain a light yellow solid product with a product quality of 14.96 g and a yield of 74.8%....
Embodiment 2
[0043] (1) Preparation of fluorine-containing block acrylate copolymer: preparation of block copolymer with molecular weight of 10,000 and block mass ratio of 7:3 terminal hydroxyl butyl methacrylate and hexafluorobutyl methacrylate
[0044] In a 100mL four-necked flask, add 0.37g (0.002mol) initiator 1-bromoethylbenzene, 14g (0.0986mol) of butyl methacrylate monomer, 0.1415g (0.000986mol) catalyst CuBr, 0.2291g (0.001972mol) ) ligand tetramethylethylenediamine, 6.02g solvent anisole, mix well. The system was evacuated and filled with nitrogen, and stirred and reacted in an oil bath at 30°C. After 24 hours, add 6 g (0.024 mol) hexafluorobutyl methacrylate, 0.0238 g (0.00024 mol) CuCl, 0.05578 g (0.00048 mol) ligand tetramethylethylenediamine to the flask, and pump again to fill with nitrogen. Stirring and reaction were continued for 24 hours in an oil bath at 50°C. After the reaction was completed, the catalyst and solvent were removed to obtain a light yellow solid product w...
Embodiment 3
[0048] (1) Preparation of fluorine-containing block acrylate copolymer: preparation of block copolymer with a molecular weight of 5000 and a block mass ratio of 9:1 terminal hydroxyl butyl methacrylate and hexafluorobutyl methacrylate
[0049] Add 0.724g (0.004mol) initiator 0-ethyl bromopropionate in 100mL four-necked flask, the butyl methacrylate monomer of 18g (0.127mol), 0.183g (0.00127mol) catalyst CuBr, 0.5852g ( 0.00254mol) ligand tris-(N,N-dimethylaminoethyl)amine (Me6 TREN), 7.2g solvent toluene, mix well. The system was evacuated and filled with nitrogen, and stirred and reacted in an oil bath at 50°C. After 18 hours, 2 g (0.008 mol) of hexafluorobutyl methacrylate, 0.0115 g (0.00008 mol) of CuBr, 0.03686 g (0.00016 mol) of the ligand tris-(N,N-dimethylaminoethyl) were added to the flask Amine (Me6 TREN), after vacuuming again and filling with nitrogen, continue to stir and react in an oil bath at 120°C for 8 hours. After the reaction is completed, after removing th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com