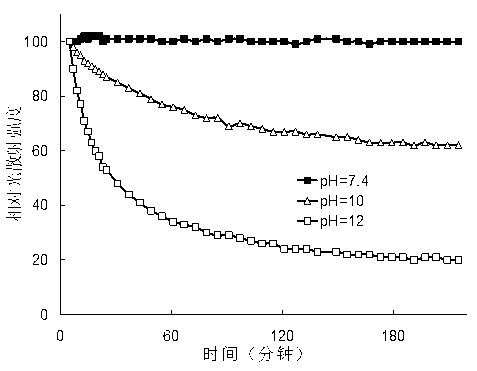
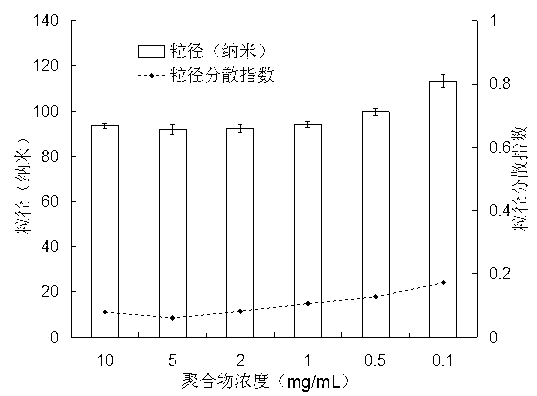
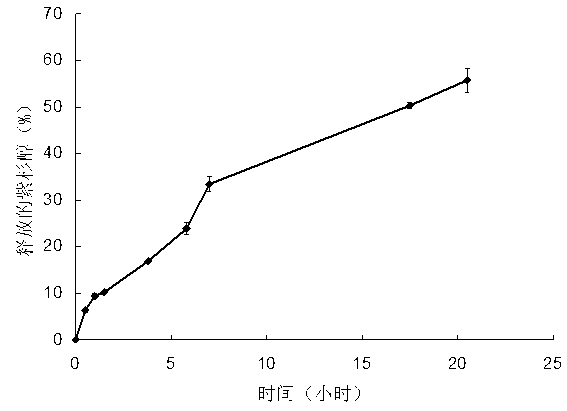
Multifunctional degradable polyasparaginate modified polymer and preparation method thereof
A polyasparagine and multifunctional technology, which is applied in the field of preparation of micelles and drug-loaded micelles, can solve the problems of being insoluble in ethanol, difficult to recover drugs, and difficult to remove, and achieves high encapsulation efficiency and method. Simple, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1-1 Preparation of polysuccinimide PSI
[0034] Add 15.9 g of phosphoric acid to 30 g of aspartic acid, under the catalysis of phosphoric acid, dehydrate by rotary steaming under reduced pressure for 2 hours at 180 ° C, stop the rotary steaming, add 200 mL of N,N-dimethylformamide (DMF) while hot, The solid was fully dissolved, and the solution was added dropwise to 1000mL distilled water to obtain a white precipitate, filtered, washed with distilled water until neutral, dried, re-dissolved with DMF, and then precipitated with distilled water again, filtered, and vacuum-dried to obtain polyamber Imide (PSI) 20.0 g. PSI with different molecular weights were synthesized by a similar method, and the molecular weight and molecular weight distribution (MWD) were measured by gel permeation chromatography (GPC). The results are shown in Table 1:
[0035] Table 1. Molecular weight characterization results of polysuccinimide (PSI)
[0036]
[0037] 1-2 activation containing...
preparation example 2
[0046] Pentanolamine Ring Opening PSI
[0047] Weigh 2 grams of the obtained polysuccinimide (PSI-3, Synthesis Example 1-1), dissolve it in 20 mL of DMF, add 5-aminopentanolamine (3.90 grams in 5 mL of DMF) dropwise under ice-cooling, Stir at room temperature for half an hour, then place it in an oil bath at 60°C for 24 hours, precipitate with anhydrous ether, and dry in vacuo to obtain 3.5 g of polymer (PHPA). The H NMR spectrum shows that PSI is completely ring-opened (there is no peak in the chemical shift between 5-6ppm on the H NMR spectrum).
[0048] 0.20 g of PHPA, 0.24 g of mPEG5k-CI (Synthesis Example 1-2) and 0.12 g of catalyst (DMAP) were placed in a round bottom flask, dissolved in 2 mL of anhydrous DMF, then heated up and reacted with magnetic stirring in an oil bath at 70°C for 48 hours . After cooling to room temperature, add 95 mg Ph-CI (Synthesis Example 1-3), continue the reaction at 70°C for 24 hours to stop, add anhydrous ether to precipitate, and dry in ...
preparation example 4
[0054] Weigh 0.2 gram of the obtained PHPA (temperature-sensitive polymer preparation example 2), 0.26 gram of mPEG5k-CI (synthesis example 1-2) and an appropriate amount of catalyst (DMAP) in a round bottom flask, add 2 mL of anhydrous DMF to dissolve, Then raise the temperature and react with magnetic stirring in an oil bath at 70°C for 24 hours; after cooling to room temperature, add 135mg Ph-CI (Synthesis Example 1-3), and continue the reaction at 70°C for 24 hours; after cooling to room temperature, add 85mg of DEAE-CI (Synthesis Examples 1-4), the reaction was continued at 70°C for 24 hours to stop, added to anhydrous ether for precipitation, and vacuum-dried to obtain 0.48 g of a polymer, which was designated as P7. Using the same method, keep the same feeding ratio of mPEG5k-CI and DEAE-CI and PHPA, change the feeding ratio of Ph-CI, and make polymer P8; In the proton NMR spectrum, the peak with a chemical shift of 7.2-7.3ppm is the proton peak of the connected benzene...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com