Co-production method and device for 2, 6-dibromo-4-nitroaniline and bromine
A technology of nitroaniline and p-nitroaniline, which is applied in two fields, can solve the problems of dilute acid and bromine added as raw materials, high raw material costs, etc., and achieve the effects of saving raw material costs, simplifying the production process, and reducing the consumption of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
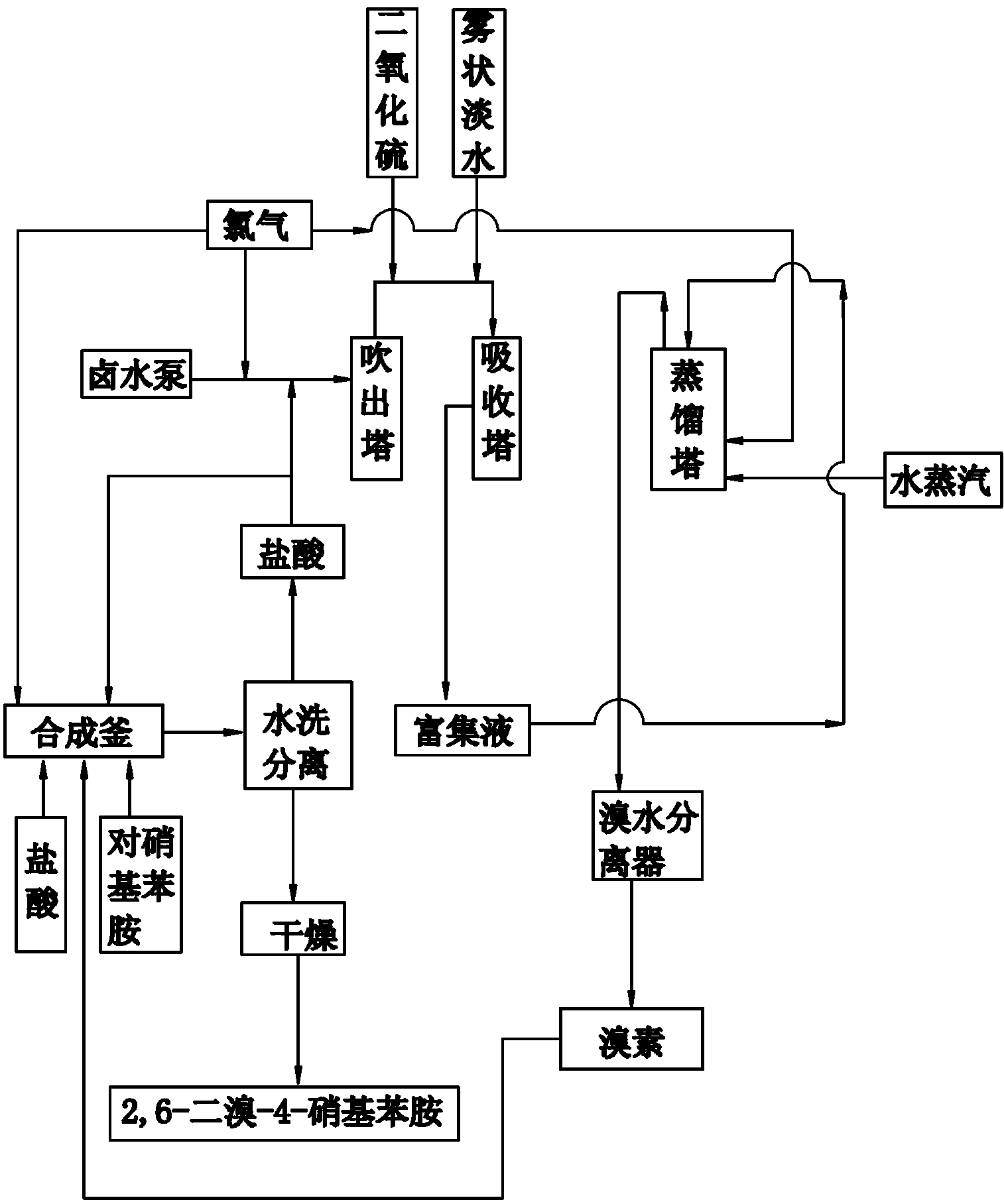
[0035] (1) Take an appropriate amount of 20% hydrochloric acid and add it to the synthesis kettle, weigh p-nitroaniline and add it to the synthesis kettle, add bromine to the synthesis kettle dropwise, the weight ratio of p-nitroaniline and bromine is 1:1.02 , a bromination reaction occurs at 40°C to generate hydrobromic acid and 2,6-dibromo-4-nitroaniline;
[0036] Feed chlorine gas into the synthesis kettle again, the molar ratio of chlorine gas and bromine added dropwise is 1:1, chlorine gas reacts with generated hydrobromic acid to generate bromine, and bromine continues to react with p-nitroaniline to generate 2,6 - Dibromo-4-nitroaniline solid and hydrochloric acid;
[0037] Send the mixture of 2,6-dibromo-4-nitroaniline solid and hydrochloric acid into a plate and frame filter, wash and separate with water, and the separated 2,6-dibromo-4-nitroaniline solid is flashed Dried in a steamer to obtain 2,6-dibromo-4-nitroaniline product;
[0038] (2) Import the hydrochloric...
Embodiment 2
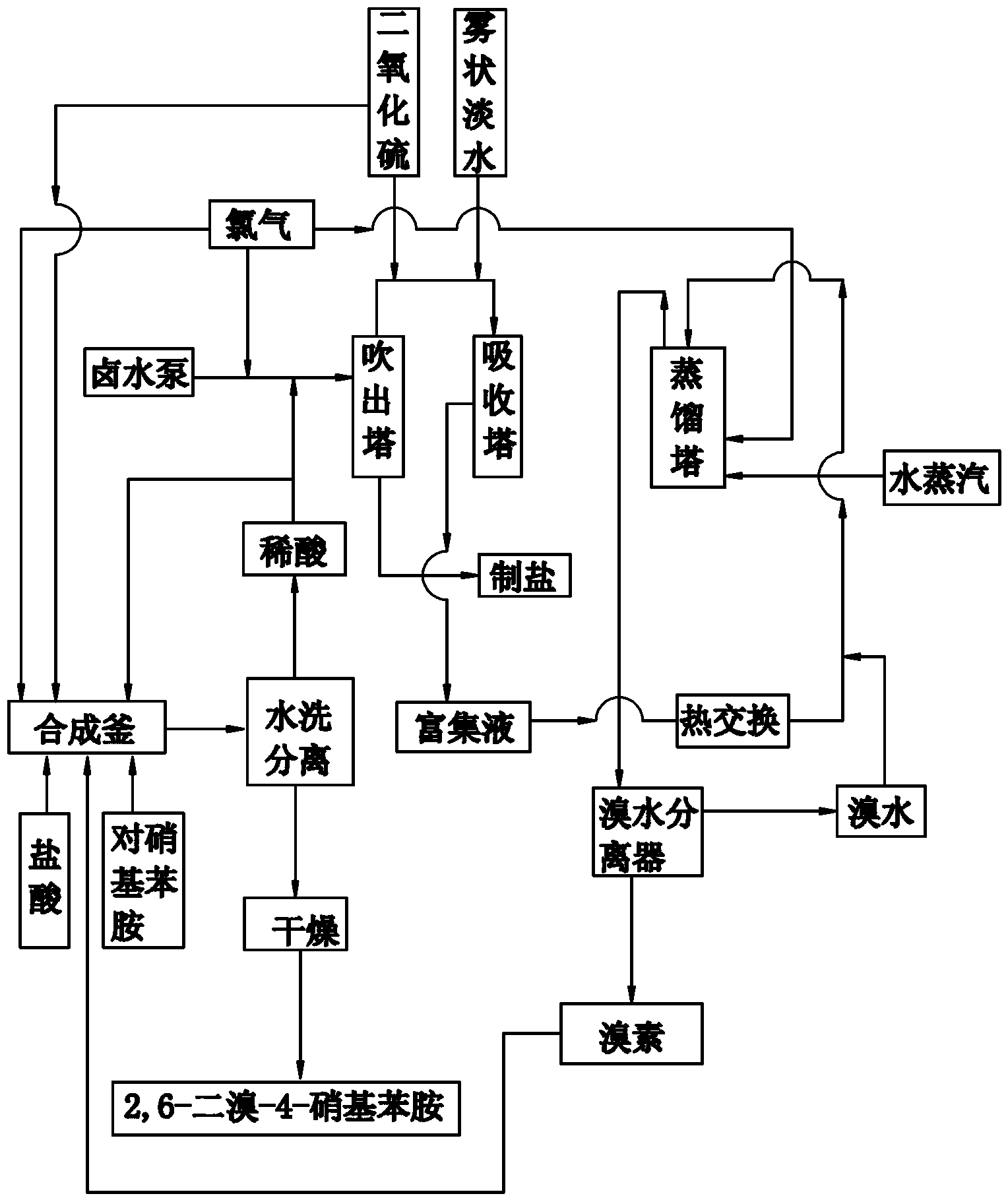
[0043] (1) Take an appropriate amount of 25% hydrochloric acid and add it to the synthesis kettle, weigh p-nitroaniline and add it to the synthesis kettle, add bromine to the synthesis kettle dropwise, the weight ratio of p-nitroaniline and bromine is 1:1.5 , a bromination reaction occurs at 45°C to generate hydrobromic acid and 2,6-dibromo-4-nitroaniline;
[0044] Feed chlorine gas into the synthesis kettle again, the mol ratio of chlorine gas and the bromine added dropwise is 1.5:1, chlorine gas reacts with the generated hydrobromic acid to generate bromine, and bromine continues to react with p-nitroaniline to generate 2,6 - Dibromo-4-nitroaniline solid and hydrochloric acid;
[0045] Send the mixture of 2,6-dibromo-4-nitroaniline solid and hydrochloric acid into a plate and frame filter, wash and separate with water, and the separated 2,6-dibromo-4-nitroaniline solid is flashed Dried in a steamer to obtain 2,6-dibromo-4-nitroaniline product;
[0046] (2) Import the hydro...
Embodiment 3
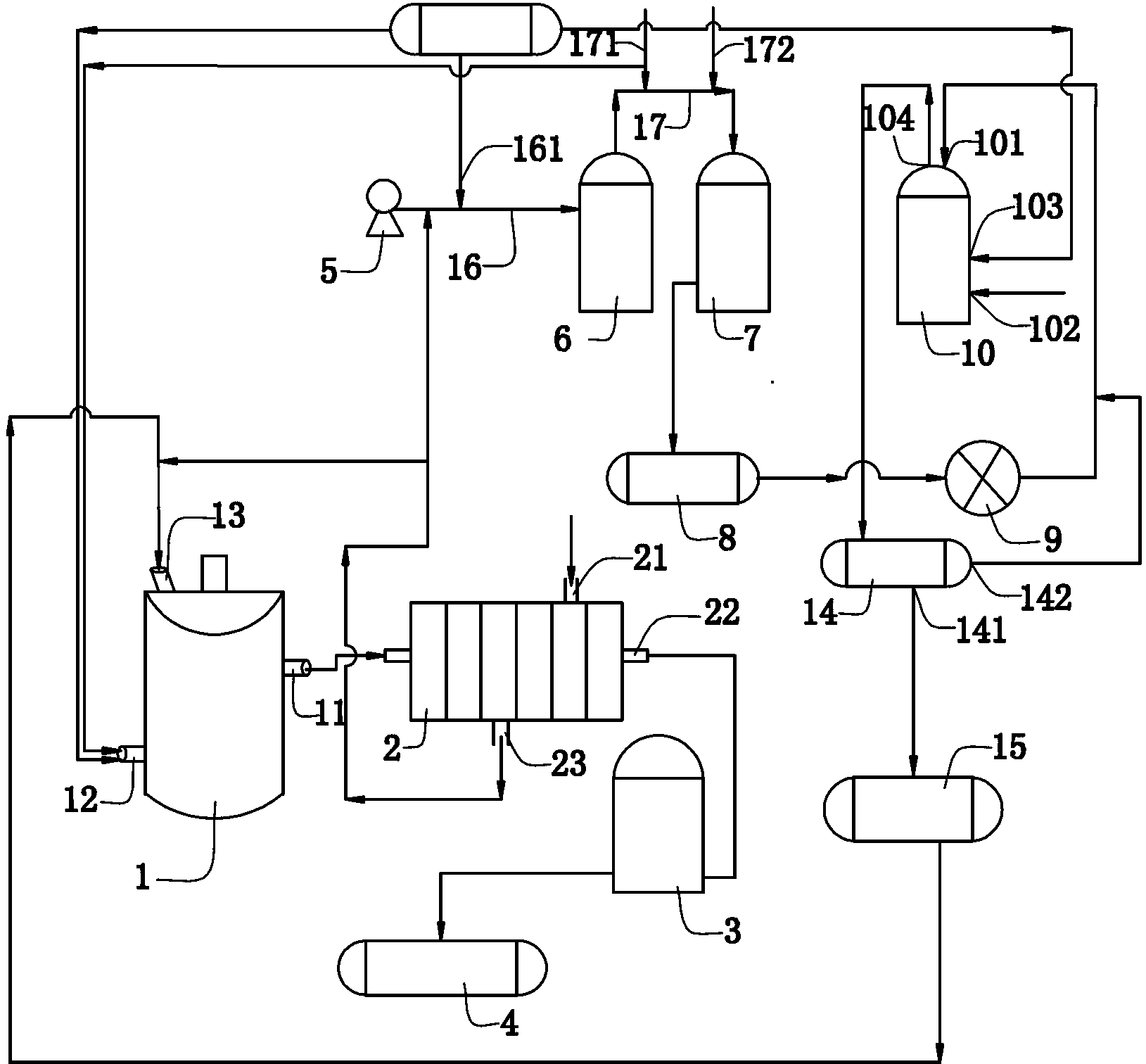
[0051] (1) Add an appropriate amount of 30% hydrochloric acid to the synthesis kettle, weigh p-nitroaniline and add it to the synthesis kettle, add bromine dropwise to the synthesis kettle, the weight ratio of p-nitroaniline and bromine is 1:2 , a bromination reaction occurs at 50°C to generate hydrobromic acid and 2,6-dibromo-4-nitroaniline;
[0052] Feed chlorine gas into the synthesis kettle again, the molar ratio of chlorine gas and the bromine added dropwise is 2:1, chlorine gas reacts with generated hydrobromic acid to generate bromine, and bromine continues to react with p-nitroaniline to generate 2,6 - Dibromo-4-nitroaniline solid and hydrochloric acid;
[0053] Send the mixture of 2,6-dibromo-4-nitroaniline solid and hydrochloric acid into a plate and frame filter, wash and separate with water, and the separated 2,6-dibromo-4-nitroaniline solid is flashed Dried in a steamer to obtain 2,6-dibromo-4-nitroaniline product;
[0054] (2) Lead the hydrochloric acid generat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com