Application of 1,4-dihydropyridine derivative as NO fluorescent probe
A technology of dihydropyridine and fluorescent probes, which can be used in fluorescence/phosphorescence, organic chemistry, material excitation analysis, etc., and can solve problems such as insufficient specificity and limited use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Synthesis of Coumarin Derivatives Based on Hantzsch Lipid Synthesis
[0025] 1. Synthesis of 1b (7-hydroxy-4-methyl-2H-chromen-2-one)
[0026]
[0027] 11g (100mmol) resorcinol, 13mL (100mmol) ethyl acetoacetate, 52mL H 3 PO 4 Add it into a round-bottomed flask, stir at room temperature for 12 hours, the solution turns into a yellow paste, pour the yellow paste into a large amount of water, and filter with suction to obtain a milky white solid. Recrystallization from ethyl acetate / petroleum ether afforded a pure white solid. 1 H NMR (400Hz CDCl 3 )δppm: 10.50(s,1H),7.60(d,1H,J=8.7Hz),6.82(q,1H,J=2.3Hz),6.70(d,1H,J=2.3Hz),6.12(d, 1H,J=0.9Hz),2.36(d,3H,J=0.9Hz).
[0028] 2. Synthesis of 1c (7-methoxy-4-methyl-2H-chromen-2-one7-methoxy-4-methylcoumarin)
[0029]
[0030] Dissolve 3.5g (22mmol) 1b with 30mL acetone, add 6.03g (43.2mmol) K 2 CO 3 , stirred for 10min, then added 3.12g (22mmol) CH 3 1, reflux reaction 6h, stop heating, heat filtration....
Embodiment 2
[0040] Example 2. Research on UV and Fluorescent Properties of Coumarin Derivatives and Fluorescent Probes
[0041] 1. The ultraviolet and fluorescent properties of coumarin derivatives:
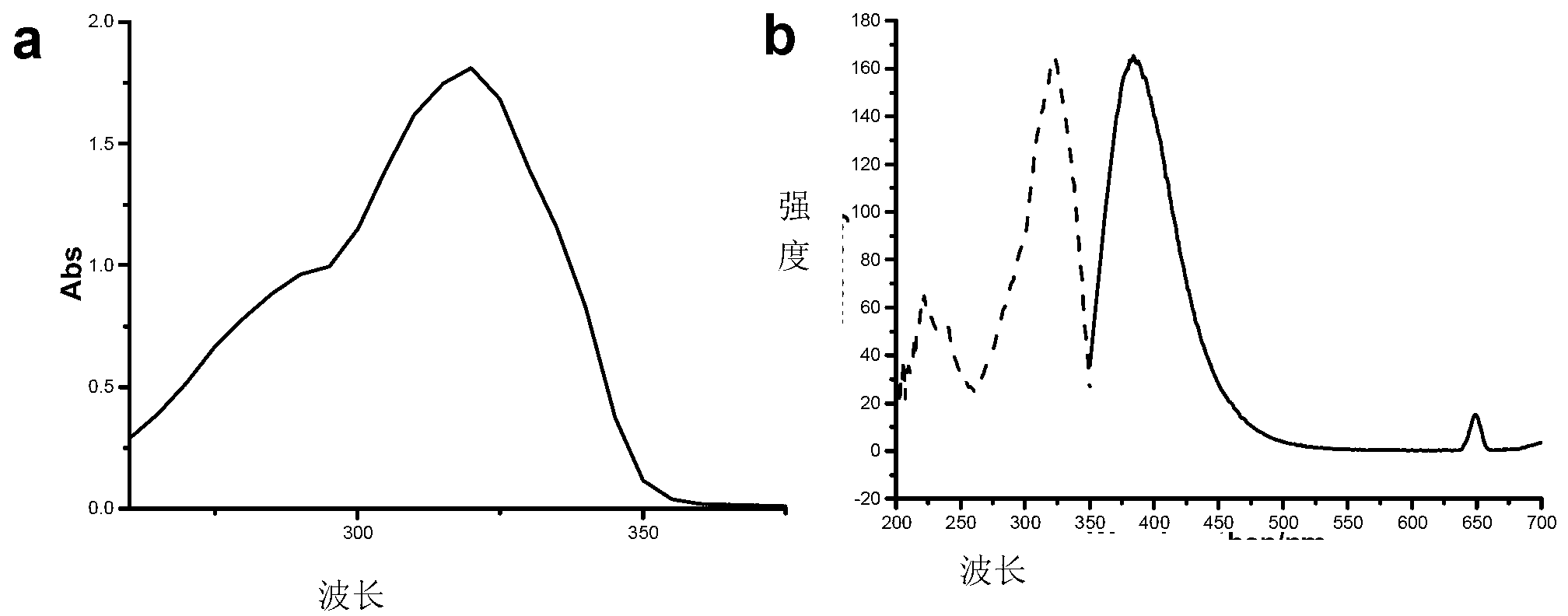
[0042] Such as figure 1 Shown, the solvent is ethanol, the ultraviolet absorption spectrum of 4-methylcoumarin 1c ( figure 1 a,c=1.0×10 -4 mol L -1 ,ε=1.81×10 4 ) and 4-methylcoumarin 1c ( figure 1 b, c=1.0×10 -6 mol L -1 ,λ ex =323nm,λ em =384nm) fluorescence spectrum.
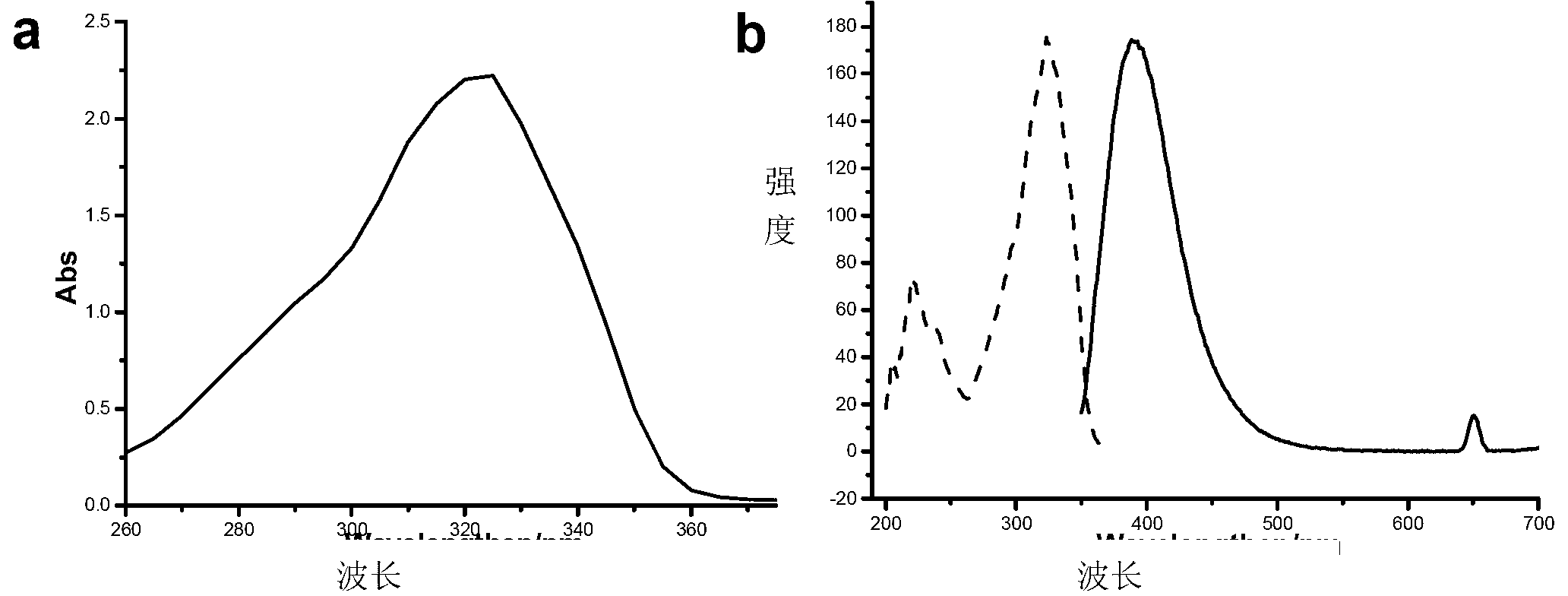
[0043] Such as figure 2 Shown, the UV absorption spectrum of 4-formyl coumarin 1d ( figure 2 a,c=1.0×10 -4 mol L -1 ,ε=2.22×10 4 ) and the fluorescence spectrum of 4-formylcoumarin 1d ( figure 2 b, c=1.0×10 -6 mol L -1 ,λ ex =324nm,λ em =389nm), the solvents are ethanol.
[0044] Such as figure 1 , figure 2 As shown, the different substituents on the 4th position of 7-methoxy-coumarin have no significant effect on its own fluorescence properties.
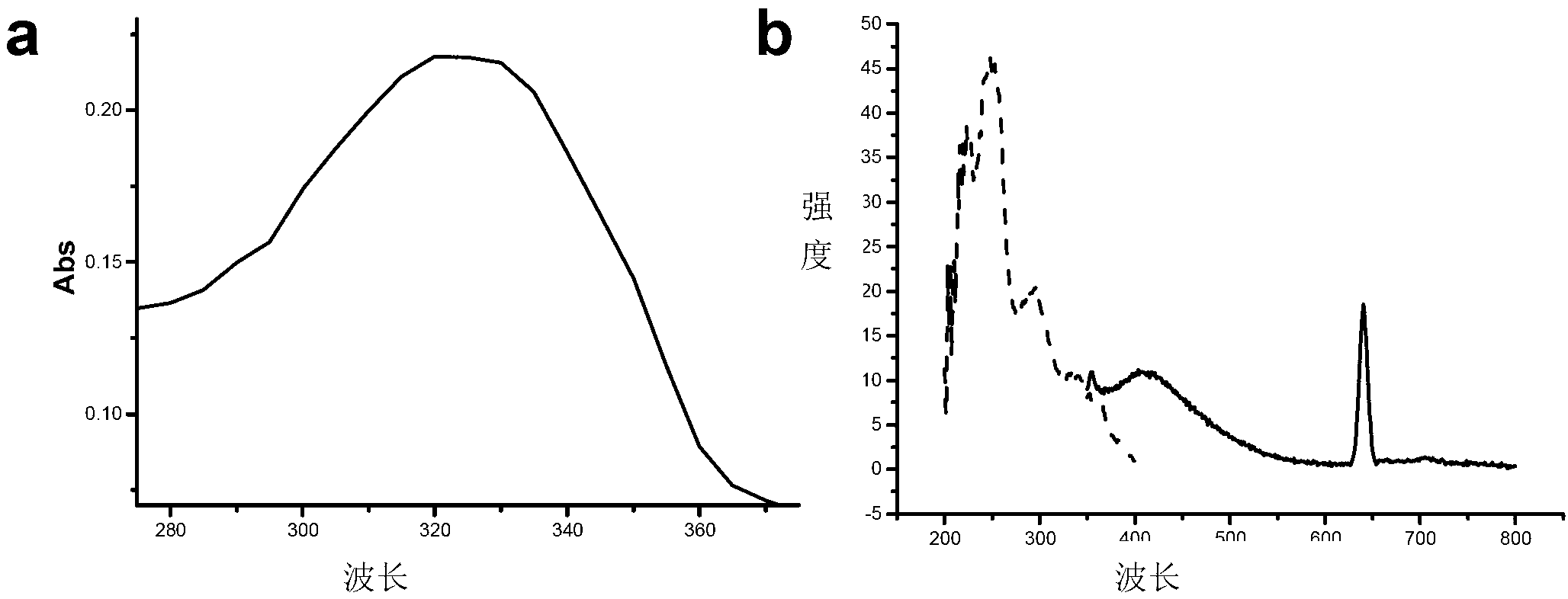
[0045] 2. The ultraviolet and fluorescent propert...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com