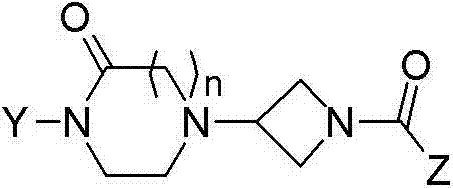
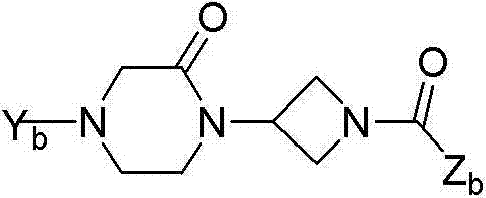
Oxopiperazine-azetidine amides and oxodiazepine-azetidine amides as monoacylglycerol lipase inhibitors
A technology of alkyl group and substituent is applied in the field of oxopiperazine-azetidine amide and oxodiazepine*-azetidine amide as monoacylglycerol lipase inhibitor, and can solve the problem of Difficulty separating side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0315] 4-pyrimidin-2-yl-1-(1-{[6-(trifluoromethyl)-1-benzothiophen-2-yl]carbonyl}azetidin-3-yl) Piperazin-2-one, compound 23
[0316]
[0317] Step A. 1-(6-Trifluoromethyl-benzo[b]thiophene-2-carbonyl)-azetidin-3-one 1e. N-Boc-azetidin-3-one 1a (171 mg, 1.0 mmol) and TFA (1 mL) were dissolved in CH 2 Cl 2 (4 mL) was stirred at room temperature for 2 h. The solution was concentrated to obtain intermediate 1b, which was used in the next step without further purification. To 6-trifluoromethyl-benzo[b]thiophene-2-carboxylic acid 1c (246 mg, 1.0 mmol) in CH 2 Cl 2 To the suspension in (10 mL) was added oxalyl chloride (0.105 mL, 1.2 mmol) followed by DMF (2 drops). The reaction mixture was stirred at room temperature for 4 h and concentrated to obtain the acid chloride 1d which was used in the next step without further purification. To 1b (1.0mmol) and Et at 0°C 3 N (0.835 mL, 6.0 mmol) in CH 2 Cl 2 (7 mL) was added 1d (1.0 mmol) in CH 2 Cl 2 (5 mL). The reactio...
example 2
[0323] 4-pyrimidin-2-yl-1-[1-({1-[4-(trifluoromethyl)phenyl]-1H-indol-5-yl}carbonyl)azetidinine- 3-yl]piperazin-2-one, compound 24
[0324]
[0325] Step A. [2-(Pyrimidin-2-ylamino)-ethyl]-carbamic acid tert-butyl ester 2a.
[0326] Within 9h, 2-bromopyrimidine 1i (600mg, 3.77mmol), N-Boc-ethylenediamine 1f (1000mg, 6.24mmol) and K 2 CO 3 (781mg, 5.66mmol) in dioxane (20mL) and H 2 The mixture in O (10 mL) was heated to reflux. The mixture was concentrated to remove most of the dioxane, and the resulting mixture was extracted with EtOAc. The organic solution was washed with NaCl aqueous solution, with Na 2 SO 4 Dried and concentrated. Purification by column chromatography (silica gel, 50% EtOAc / heptane) afforded Intermediate 2a (770 mg) as a white solid.
[0327] Step B. 3-[2-(Pyrimidin-2-ylamino)-ethylamino]-azetidine-1-carboxylic acid tert-butyl ester 2b .
[0328] Intermediate 2a (610 mg, 2.56 mmol) was dissolved in TFA (2 mL) and CH 2 Cl 2 (8 mL) wa...
example 3
[0334] 1-pyrimidin-2-yl-4-[1-({1-[4-(trifluoromethyl)phenyl]-1H-indol-5-yl}carbonyl)azetidinine- 3-yl]piperazin-2-one, compound 3
[0335]
[0336] Step A. tert-butyl 3-oxo-4-pyrimidin-2-yl-piperazine-1-carboxylate 3b .
[0337] To 1-Boc-3-oxopiperazine 3a (390 mg, 1.95 mmol) in DMF (5 mL) was added NaH (60% in oil, 97 mg, 2.44 mmol) at 0°C. The reaction was stirred at 0° C. for 25 min before adding a solution of 2-bromopyrimidine 1i (465 mg, 2.92 mmol) in DMF (2 mL). The reaction was slowly warmed to room temperature overnight. Add H to the reaction mixture 2 0, and the resulting mixture was extracted with EtOAc. The organic solution was washed with NaCl aqueous solution, with Na 2 SO 4 Dried and concentrated. By column chromatography (silica gel, 3% MeOH / CH 2 Cl 2 ) to obtain 3b (120 mg) as a yellow oil.
[0338] Step B. 3-(3-Oxo-4-pyrimidin-2-yl-piperazin-1-yl)-azetidine-1-carboxylic acid tert-butyl ester 3c .
[0339] Intermediate 3b (120 mg, 0.43 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com