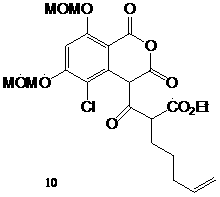
2,4-dihydroxy-5,6-substituted-1-halogenated benzene derivatives, their synthesis method and application
A synthesis method and technology of halogenated benzene, applied in the field of application of 2,4-dihydroxy-1-halogenated benzene derivatives, to achieve good development and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
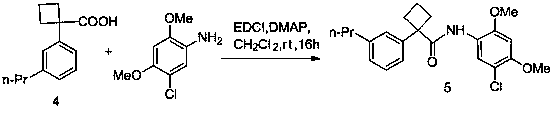
[0017] The first type of compound in the patent of the present invention can be synthesized by the following scheme to synthesize the key intermediate compound 2,4-dimethoxy-5-chlorophenylboronic acid (4) and the target compound.
[0018]
[0019] Compound (2): When R 3 When =H, add 2,4-dimethoxybenzene and THF into the round bottom flask, add dibromohydantoin (DBDMH) at room temperature, and continue stirring at room temperature for 6h after the addition is complete. TLC monitoring, after the reaction was completed, the solvent was evaporated in vacuo, water was added to the flask, the mixture was extracted with ethyl acetate, dried over anhydrous sodium sulfate, evaporated to dryness to obtain colorless transparent crystal compound (2), the yield was 96% ; 1 HNMR (300MHz, CDCl 3 ):7.39(1H,d,J=9.0Hz,Ar-H),6.47(1H,s,Ar-H),6.38(1H,d,J=9.0Hz,Ar-H),3.85(3H,s ,OCH 3 ),3.78(3H,s,OCH 3 ).
[0020] Compound (3): In a round bottom flask, add compound (2), NCS and acetonitrile...
Embodiment 2
[0036] The second type of compound of the present invention can be synthesized by the following scheme to synthesize the key intermediate compound (8) and the target compound.
[0037]
[0038] Compound (2): add the raw material p-chlorophenol, anhydrous potassium carbonate solid, and acetone into a 500 mL round-bottomed flask, add methyl iodide, install a reflux condensing device, and heat to reflux. The reaction was followed by TLC and was complete. Then, the solvent and residual iodomethane were evaporated under reduced pressure, the obtained solid was extracted with ethyl acetate, the separated organic phase was washed with saturated brine, and dried over anhydrous sodium sulfate. After the solvent was evaporated under reduced pressure, the crude pale yellow oil was separated and purified by silica gel column to obtain compound (2) as a colorless oil with a yield of 98%. 1 HNMR (300MHz, CD 3 Cl 3 ): δ3.80(3H,s),3.88(3H,s),6.43(1H,dd,J=9Hz,3Hz),6.50(1H,d,J=3Hz),7.23(1...
Embodiment 3
[0060] The third type of compound of the present invention can be synthesized by the following scheme to synthesize the key intermediate compound (2) and the target compound.
[0061]
[0062] Compound (2): Dissolve iso-xylylene ether and n-chlorosuccinimide in acetonitrile, heat the system under reflux for 17 hours, evaporate the solvent, and pass through a column with petroleum ether to obtain a colorless oily compound (2). ) with a yield of 90%. 1 HNMR (300MHz, CDCl 3 )δ:7.26-7.23(m,1H),6.51(d,1H,J=2.7Hz),6.43(dd,1H,J=2.7,8.7Hz),3.87(s,3H),3.79(s,3H ).
[0063] Compound (3): 1-adamantanol was added to the mixture of compound (2) and trifluoroacetic acid, stirred at room temperature for 0.5 hour, 15 mL of water was added to the reaction system, then extracted with ethyl acetate, and the organic compounds were combined. The phase was dried with anhydrous sodium sulfate, evaporated to dryness, and purified by thin-layer chromatography using petroleum ether as a developin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com