Peptide fragment-containing cyanoacrylate derivative as well as preparation method and use thereof
A technology of cyanoacrylic acid and anthracene cyanoacrylic acid, which is applied in the field of α-cyanoacrylic acid derivatives, can solve the problems of poor skin compliance, high polymer hardness, and slow polymer degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
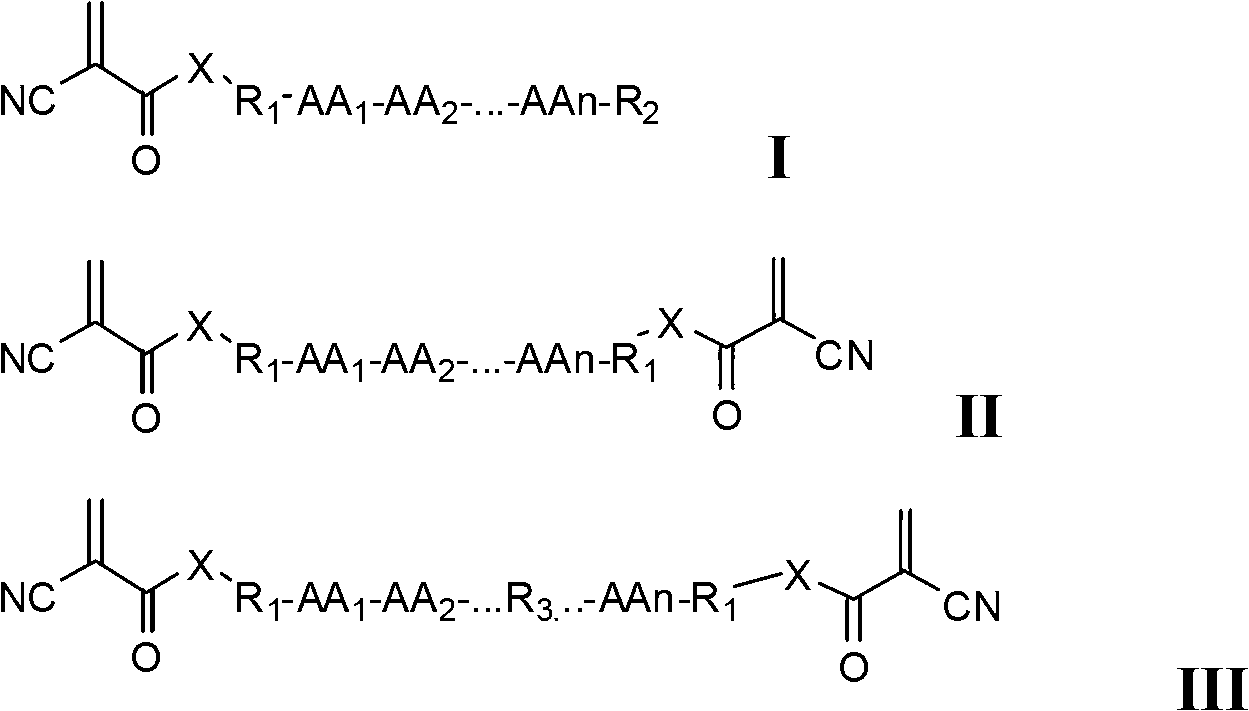
[0051] The cyanoacrylic acid derivative molecules containing peptide fragments represented by formula I, formula II, and formula III can rapidly polymerize the ethylenic bonds in the monomer molecules under the condition of a very small amount of anion triggering, resulting in adhesive adhesive ability and obtain polymers. These molecules can be used alone or as a polymer matrix component, and can also be combined with other α-cyanoacrylate molecules to form a polymer matrix. Other α-cyanoacrylate molecules may include, but are not limited to: ethyl α-cyanoacrylate, n-butyl α-cyanoacrylate, n-octyl α-cyanoacrylate, isobutyl α-cyanoacrylate , α-isooctyl cyanoacrylate, α-ethylene glycol cyanoacrylate, polyethylene glycol α-cyanoacrylate, α-polyethylene glycol monomethyl ether (mPEG) cyanoacrylate, bis-alpha - PEG diester cyanoacrylate, alkyl glycol bis-alpha-cyanoacrylate, bis-alpha-cyanoacrylic acid (polylactic acid (PLA)) diester, bis-alpha-cyanoacrylic acid (polyglycolic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com