Recombinant fusion protein of IL3 and Lidamycin, preparation method and application thereof
A technology of fusion protein and protein, which is applied in the field of proteomics, biopharmaceuticals, and oncology. It can solve the problems of high molecular weight, difficult internalization, immunogenicity, poor stability, etc., and achieve the goal of overcoming lethal side effects, not easy to degrade, and good The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
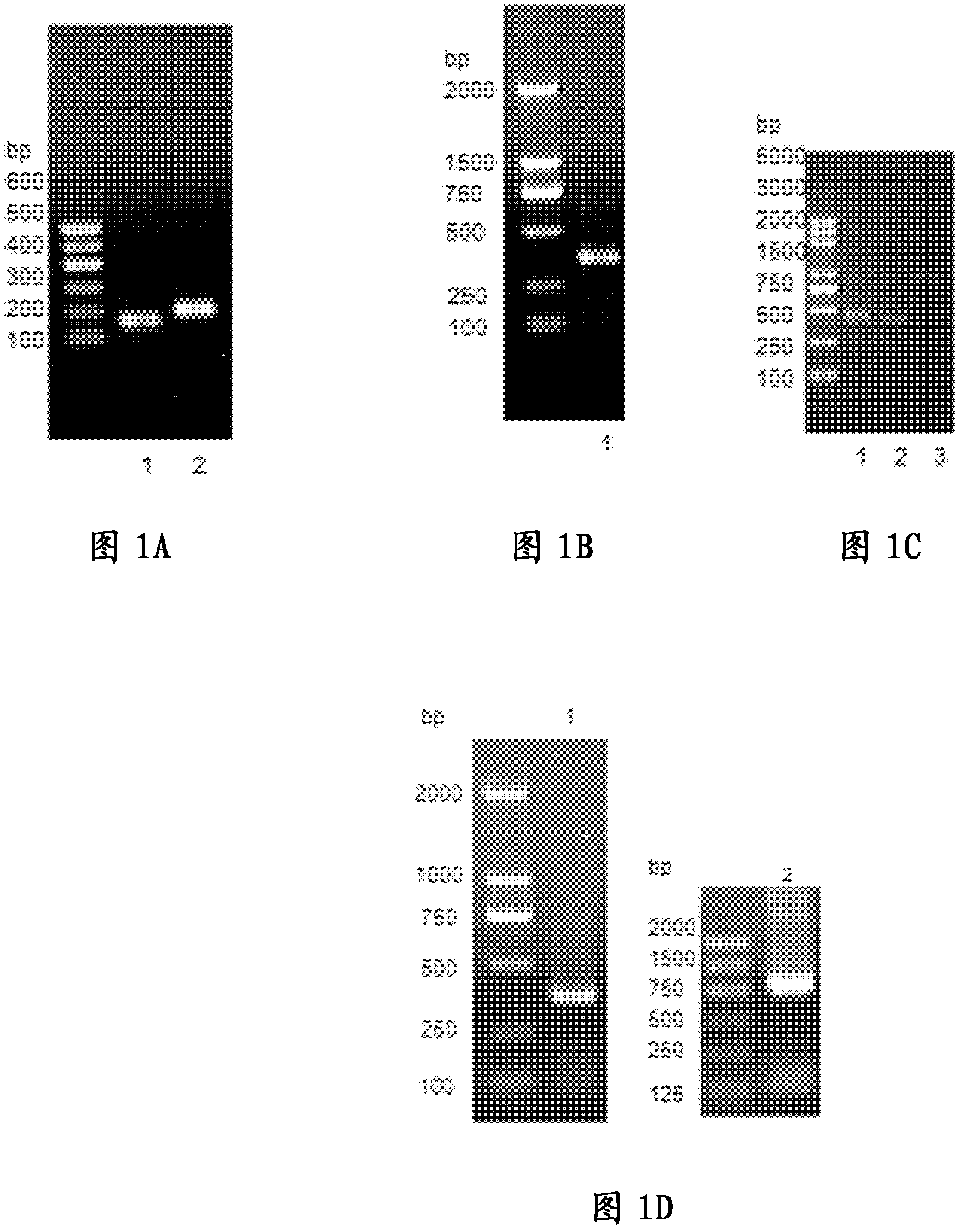
[0070] Example 1 Construction of recombinant expression plasmids pET28a IL3-LDP and pET28a mIL3-LDP
[0071] PCR amplification of IL3:
[0072] Take about 10ml of peripheral venous blood from normal people, add lymphocyte separation solution after anticoagulant treatment, absorb buffy coat phase, wash twice with 1×PBS, culture with RPMI1640 medium containing 10% fetal bovine serum, and add IL -2 and CD3 / p-gp bifunctional antibodies stimulate cell proliferation. The total RNA of normal human peripheral blood buffy coat cells was extracted, and the corresponding cDNA was obtained by reverse transcription PCR (RT-PCR) as a template to obtain IL3 that was completely consistent with the cDNA sequence of the human IL-3 core coding region retrieved from Genbank. The full length of the gene sequence. The PCR primers were synthesized by Shanghai Yingjun Company, and the corresponding enzyme cutting sites were respectively introduced.
[0073] Specifically, the cDNA of human peripher...
Embodiment 2
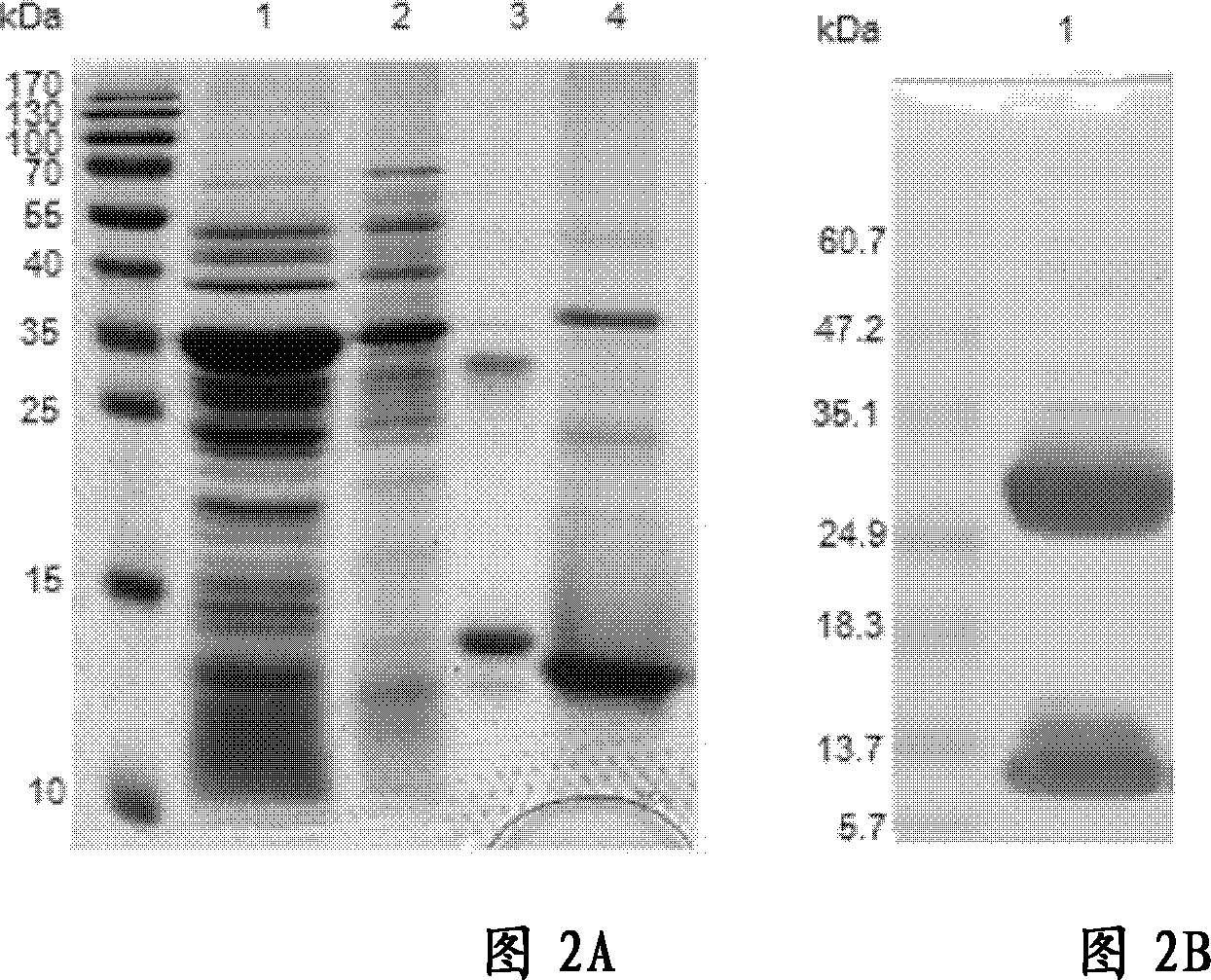
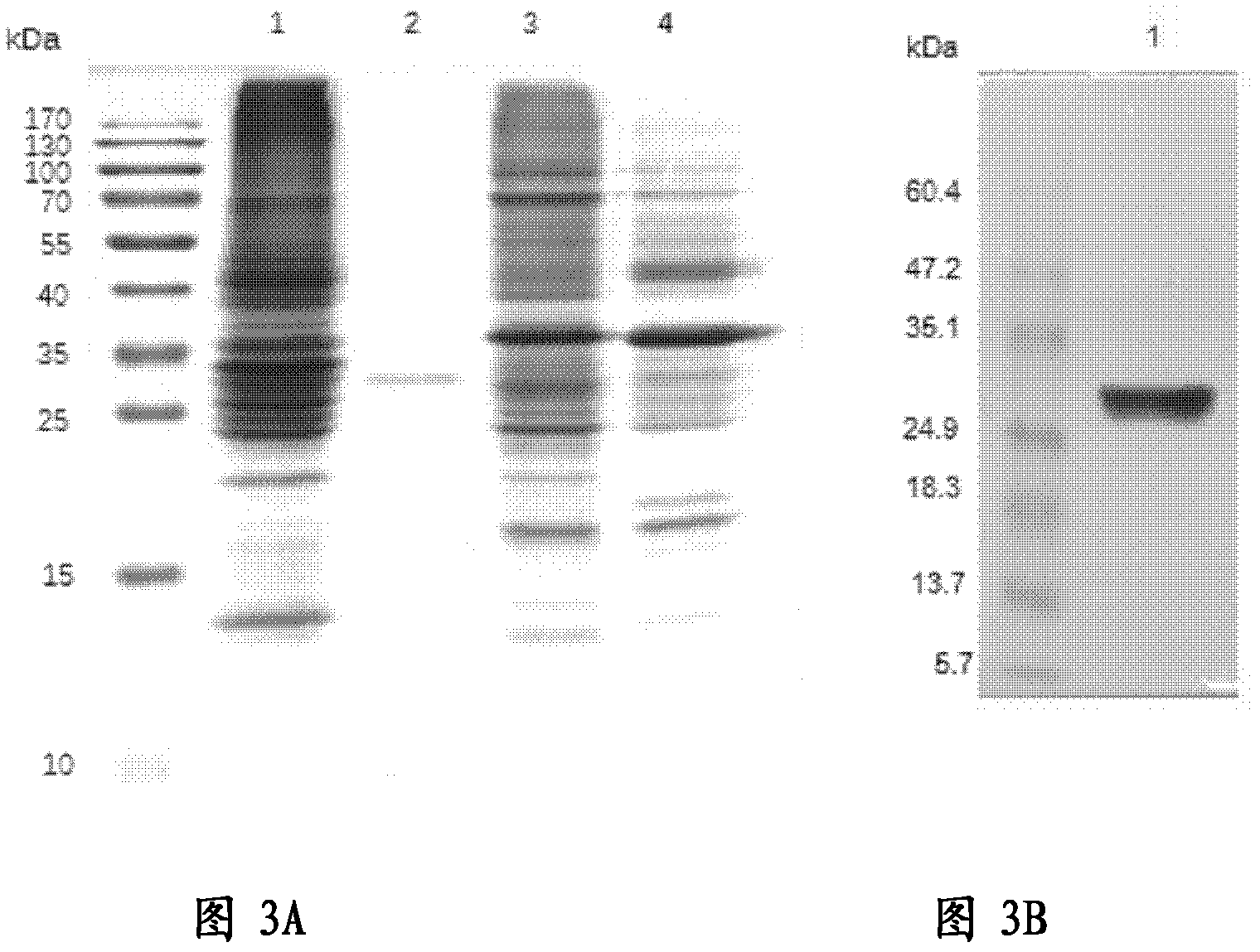
[0107] Example 2 Expression and verification of IL3-LDP and mIL3-LDP
[0108] 2.1 Expression of IL3-LDP and mIL3-LDP
[0109] The single bacterium colony of Escherichia coli BL21 containing plasmid pET28a IL3-LDP and pET28a mIL3-LDP obtained in Example 1 was inoculated in 5ml of 2×YT medium containing kanapenicillin (Kan) 50 μg / ml, at a constant temperature In a shaker box at 37°C, 200rpm, shake culture overnight; transfer 500ml of 2×YT medium containing Kan 100μg / ml (1L of 2×YT medium contains 1.6% tryptone, 1.0% yeast extract, 0.5% Sodium chloride, pH 7.4), 37°C, 200rpm, shaking culture for 8h, then centrifuge at 6000rpm, 4°C for 10 minutes on a low-temperature high-speed vacuum centrifuge to collect the bacteria, resuspend the bacteria in 1000ml containing Kan 100μg / ml and 1mM IPTG 2×YT medium, 30°C, 200rpm, shaking culture for 4h; 8,000rpm, 4°C centrifugation for 10 minutes to collect the bacteria, frozen in -20°C refrigerator for later use.
[0110] Thaw the frozen bact...
Embodiment 3
[0113] Example 3 Immunological activity of IL3-LDP and mIL3-LDP
[0114] The in vitro immunofluorescence binding activity of IL3-LDP and mIL3-LDP was determined by flow cytometry. Will 1×10 6 TF-1 cells per ml were resuspended in 100 μL PBS solution containing different concentrations of FITC-labeled anti-CD123-perCP5.5 antibody or IL3-LDP and mIL3-LDP obtained in Example 2, placed at 4°C for 1 h, centrifuged at 2000 g After 10 minutes, the supernatant was discarded, washed three times with PBS, and the positive rate of anti-CD123 binding to TF-1 cells was determined by FACS. Prove that the same concentration of anti-CD123 antibody and IL3-LDP and mIL3-LDP have basically the same binding activity to TF-1 cells, IL3-LDP and mIL3-LDP fusion protein retains the ability to specifically bind to the target antigen ( Figure 4 ).
[0115] After the flow cytometry sample is ready, centrifuge at 2000g for 10 minutes, discard the supernatant; add 4% paraformaldehyde solution for fixa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com