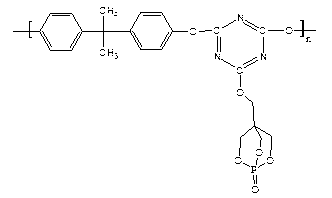
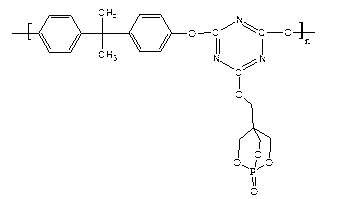
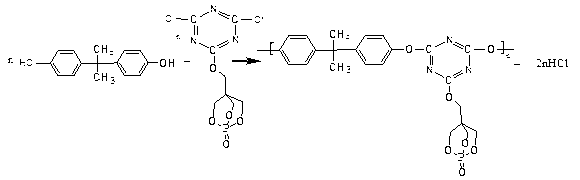
Higher molecular weight type intumescent flame retardant containing triazine structure and synthetic method thereof
An intumescent flame retardant and high molecular weight technology, applied in the field of intumescent flame retardants, can solve the problems of compatibility, thermal stability to be further improved, low molecular weight, etc., to maintain the original performance, high molecular weight, use safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Step 1: Phosphorus oxychloride reacts with pentaerythritol to obtain oxo-4-hydroxymethyl-2,6,7-trioxa-1-phosphabicyclo[2,2,2]octane (PEPA).
[0030] In a 250ml three-neck flask equipped with a thermometer, a constant pressure funnel, and a reflux condenser, add 0.25mol of pentaerythritol and 0.425mol of dioxane solvent, raise the temperature to 80°C, and dropwise add 0.25mol of phosphorus oxychloride in 2 hours. The temperature was raised to 100°C, and the reaction was carried out with nitrogen gas for 4 hours. Stand still, cool, precipitate white crystals, and filter with suction. Wash with 0.5 mol of dioxane and 1 mol of absolute ethanol respectively. Vacuum dry at 70°C for 12h.
[0031] The second step: PEPA reacts with cyanuric chloride to obtain a substituted product of cyanuric chloride.
[0032] 0.033mol PEPA is dissolved in 0.031mol acetonitrile, and 0.033mol cyanuric chloride is dissolved in 0.046mol acetonitrile. PEPA and acetonitrile solution and 0.05mol ...
Embodiment 2
[0036] Step 1: Phosphorus oxychloride reacts with pentaerythritol to obtain oxo-4-hydroxymethyl-2,6,7-trioxa-1-phosphabicyclo[2,2,2]octane (PEPA).
[0037] In a 250ml three-neck flask equipped with a thermometer, a constant pressure funnel, and a reflux condenser, add 0.3mol of pentaerythritol and 0.425mol of dioxane solvent, raise the temperature to 85°C, add 0.25mol of phosphorus oxychloride dropwise in 4 hours, and raise the temperature to 105 ℃, reacted with nitrogen gas for 6h, let stand, cooled, precipitated white crystals, filtered with suction, washed with 0.5mol dioxane and 1mol anhydrous ethanol respectively, and dried in vacuum at 70°C for 12h to obtain oxo-4-hydroxymethyl- 2,6,7-Trioxa-1-phosphabicyclo[2,2,2]octane (PEPA), molecular weight 179.98.
[0038] Infrared data: wave number 3410cm -1 The -OH absorption peak is at 2970cm -1 at -CH 2 - Absorption peak, 1301cm -1 The place is P=O absorption peak, 1022cm -1 and 990cm -1 Nearby is the P-O-C absorption pea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com