Method for preparing potassium nitrate by utilizing nitric acid and potassium chloride
A technology of potassium nitrate and potassium chloride, applied in the field of preparing potassium nitrate, which can solve the problems of low product purity, loss of organic phase, harsh production process conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
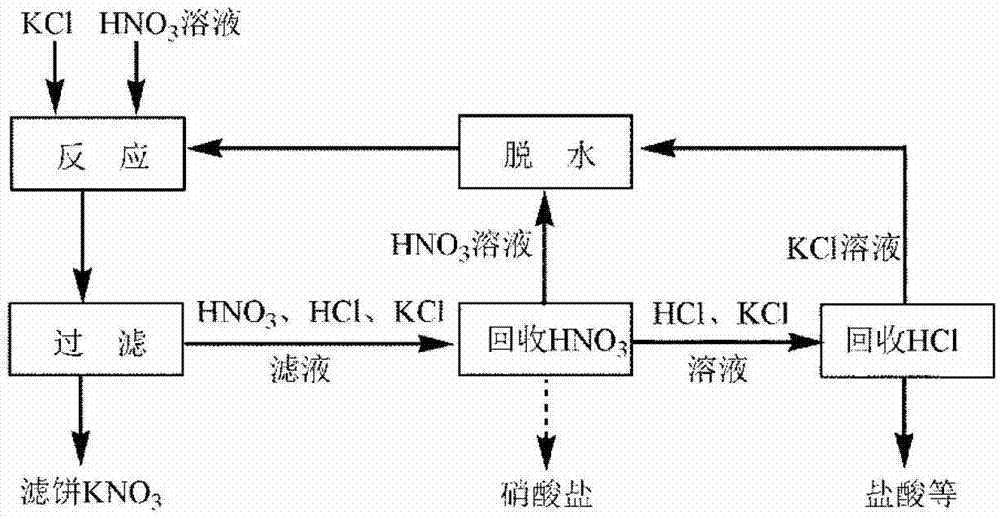
[0022] according to figure 1 As shown in the flowchart, 100g of potassium chloride and 20mL of water are mixed to form a wet slurry, and the wet slurry is reacted with 180mL of nitric acid with a mass concentration of 50% at 35°C for 2h, the feed liquid is filtered, the filter cake is washed with 10mL of water, and the filtrate is total The volume is about 185mL, and the purity of potassium nitrate in the filter cake is 78%. The nitric acid in the filtrate was extracted with 400mL methyl isobutyl ketone, and the extraction rate of nitric acid was 96%. Back-extract the organic phase with 400mL water to obtain about 3% nitric acid solution. After the filtrate was extracted for the first time, continue to extract the hydrochloric acid therein with 300mL methyl isobutyl ketone, the extraction rate of hydrochloric acid reached 93%, and the filtrate became 180mL of about 1.3mol / L potassium chloride solution. Combine the organic phases of the two extractions, back-extract with 400m...
Embodiment 2
[0024] according to figure 1 As shown in the flow chart, react 110g of potassium chloride solid with 400mL of nitric acid with a mass concentration of 36% at 0°C for 1h, filter the feed liquid, wash the filter cake with 25mL of water, the total volume of the filtrate is about 384mL, and the purity of potassium nitrate in the filter cake is 94%. The nitric acid in the filtrate was extracted by using 500mL tributyl phosphate whose volume fraction was 30% tributyl phosphate and kerosene mixture, and the extraction rate of nitric acid was 99%. Back-extract the organic phase with 400mL of 1mol / L potassium chloride solution to obtain about 4% nitric acid solution. After the first extraction of the filtrate, continue to extract the hydrochloric acid with 200mL trioctylamine and n-octanol mixture with a volume fraction of 45% trioctylamine, the extraction rate of hydrochloric acid reaches 99%, and the filtrate becomes 375mL about 1.2mol / L of potassium chloride solution. Back-extrac...
Embodiment 3
[0026] according to figure 1 As shown in the flowchart, 105g of potassium chloride and 30mL of water are mixed to form a wet slurry, and the wet slurry is reacted with 160mL of nitric acid with a mass concentration of 65% at 5°C for 0.5h, the feed liquid is filtered, the filter cake is washed with 20mL of water, and the filtrate The total volume is about 180mL, and the purity of potassium nitrate in the filter cake is 86%. The nitric acid in the filtrate was extracted by using 90mL trioctylamine and n-octanol mixed solution with a volume fraction of 50% trioctylamine, and the extraction rate of nitric acid was 99%. Back-extract the organic phase with 90 mL of 0.5 mol / L dilute potassium hydroxide solution to obtain a potassium nitrate solution. After the first extraction, the filtrate was further extracted with 360mL trialkylphosphine oxide, the extraction rate of hydrochloric acid reached 95%, and the filtrate became 168mL potassium chloride solution of about 1.2mo / L. The or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com