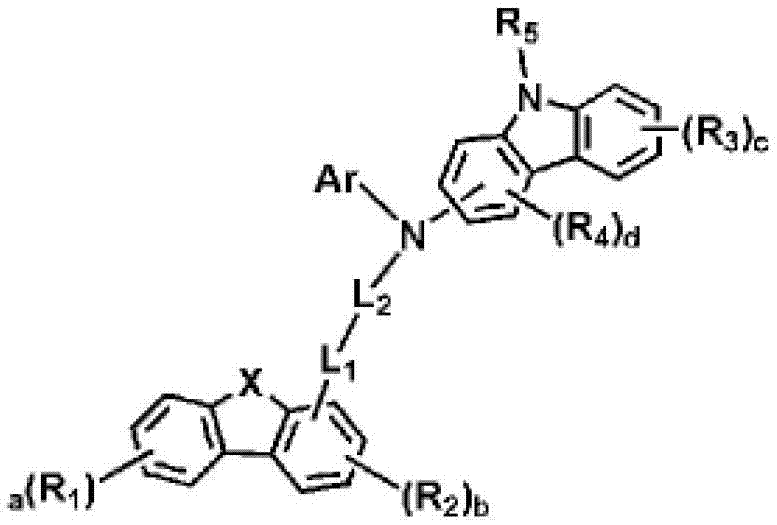
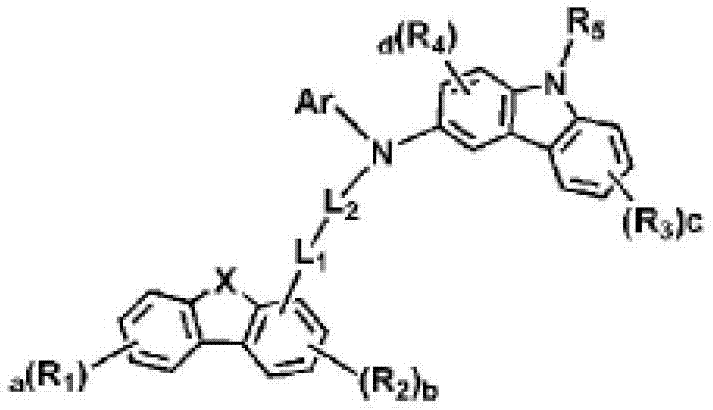
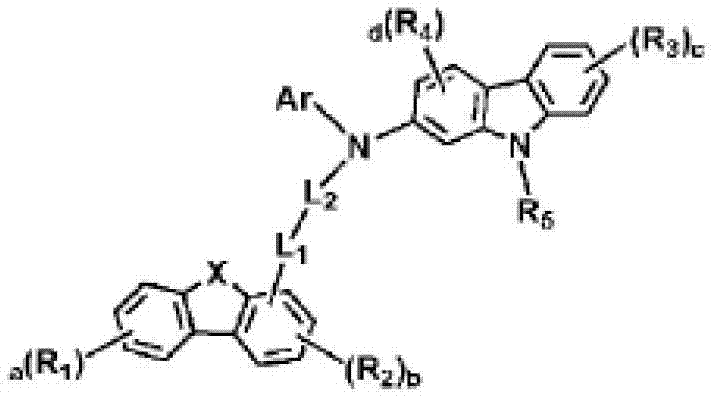
Novel organic electroluminescent compounds and organic electroluminescent devices including the same
An electroluminescence and compound technology, applied in the field of organic electroluminescence devices, which can solve the problems of high amorphousness and inability to provide durable luminous efficiency of organic EL devices.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0057] [Preparation Example 1] Preparation of Compound 1
[0058]
[0059] Preparation of compound 1-1
[0060] Add 3-bromo-9-phenyl-9H-carbazole 30g (93.1mmol), aniline 26mL (279.3mmol), Pd(OAc) 2 0.63g(2.79mmol), P(t-Bu) 3 4.3mL (9.31mmol), Cs 2 CO 3 76g (232.8mmol) and toluene 700mL, then heated and stirred at 120°C for 12 hours. After the reaction was completed, the resulting substance was washed with distilled water and extracted with ethyl acetate. with MgSO 4 The organic layer was dried and the solvent was removed with a rotary evaporator, followed by purification using column chromatography to obtain Compound 1-1 (20 g, 59.8 mmol).
[0061] Preparation of compound 1-2
[0062] Add 10.5g (46mmol) of 4-dibenzothiophene boronic acid, 32.6g (138mmol) of 1,4-dibromobenzene, Pd(PPh 3 ) 4 0.53g (0.46mmol), 1M Na 2 CO 3 69mL (69mmol) and toluene 600mL, and then stirred at reflux at 70°C for 2 hours. After the reaction was completed, the resulting substance w...
preparation example 2
[0066] [Preparation Example 2] Preparation of Compound 9
[0067]
[0068] Preparation of compound 2-1
[0069] In the reactor, add 2-naphthol 20.0g (138.8mmol), NaHSO 3 28.8g (277.4mmol), 400mL of distilled water, 31.2mL (166.4mmol) of 4-bromophenylhydrazine, and then heated to 120°C. After 12 hours, distilled water was added thereto, and the resulting solid was filtered under reduced pressure. The obtained solid was put into an aqueous hydrochloric acid solution, followed by heating at 100°C. After 1 hour, the resulting material was extracted with dichloromethane, washed with distilled water and aqueous NaOH. Column separation was then performed to obtain compound 2-1 (9.2 g, 31.0 mmol).
[0070] Preparation of Compound 2-2
[0071] Add compound 2-19.2g (31.0mmol), Cu2.0g (31.0mmol), 18-crown-60.4g (1.6mmol), K 2 CO 3 12.8g (93.2mmol) and 100mL of 1,2-dichlorobenzene were then stirred at reflux at 180°C for 12 hours. The resulting material was cooled at room t...
preparation example 3
[0079] [Preparation Example 3] Preparation of Compound 27
[0080]
[0081] Preparation of compound 3-1
[0082] Add 5.4g (44mmol) of phenylboronic acid, 18.9g (66mmol) of 1,4-dibromonaphthalene, Pd(PPh 3 ) 4 2.0g (1.76mmol), Na 2 CO 3 14.0 g (132 mmol), 200 mL of toluene, and 100 mL of ethanol were then stirred at reflux at 120° C. for 12 hours. After the reaction was completed, the resulting substance was washed with distilled water and extracted with ethyl acetate. with MgSO 4 The organic layer was dried and the solvent was removed with a rotary evaporator, followed by purification using column chromatography to obtain compound 3-1 (9.1 g, 32.14 mmol).
[0083] Preparation of Compound 3-2
[0084] Add compound 3-126.4g (93.1mmol), compound 2-372.1g (279.3mmol), Pd(OAc) in the reactor 2 0.63g(2.79mmol), P(t-Bu) 3 4.3mL (9.31mmol), Cs 2 CO 3 76g (232.8mmol) and toluene 1000mL were then heated and stirred at 120°C for 12 hours. After the reaction was complet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com