Lithium iron phosphate secondary structure, preparation method of the lithium iron phosphate secondary structure, and lithium ion battery
一种磷酸铁锂、二次结构的技术,应用在二次电池、电池电极、结构零件等方向,能够解决增加工艺步骤和条件、锂离子扩散速度慢、多导电剂和粘结剂等问题,达到提高堆积密度、易于制浆和成膜、缩短扩散路径的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
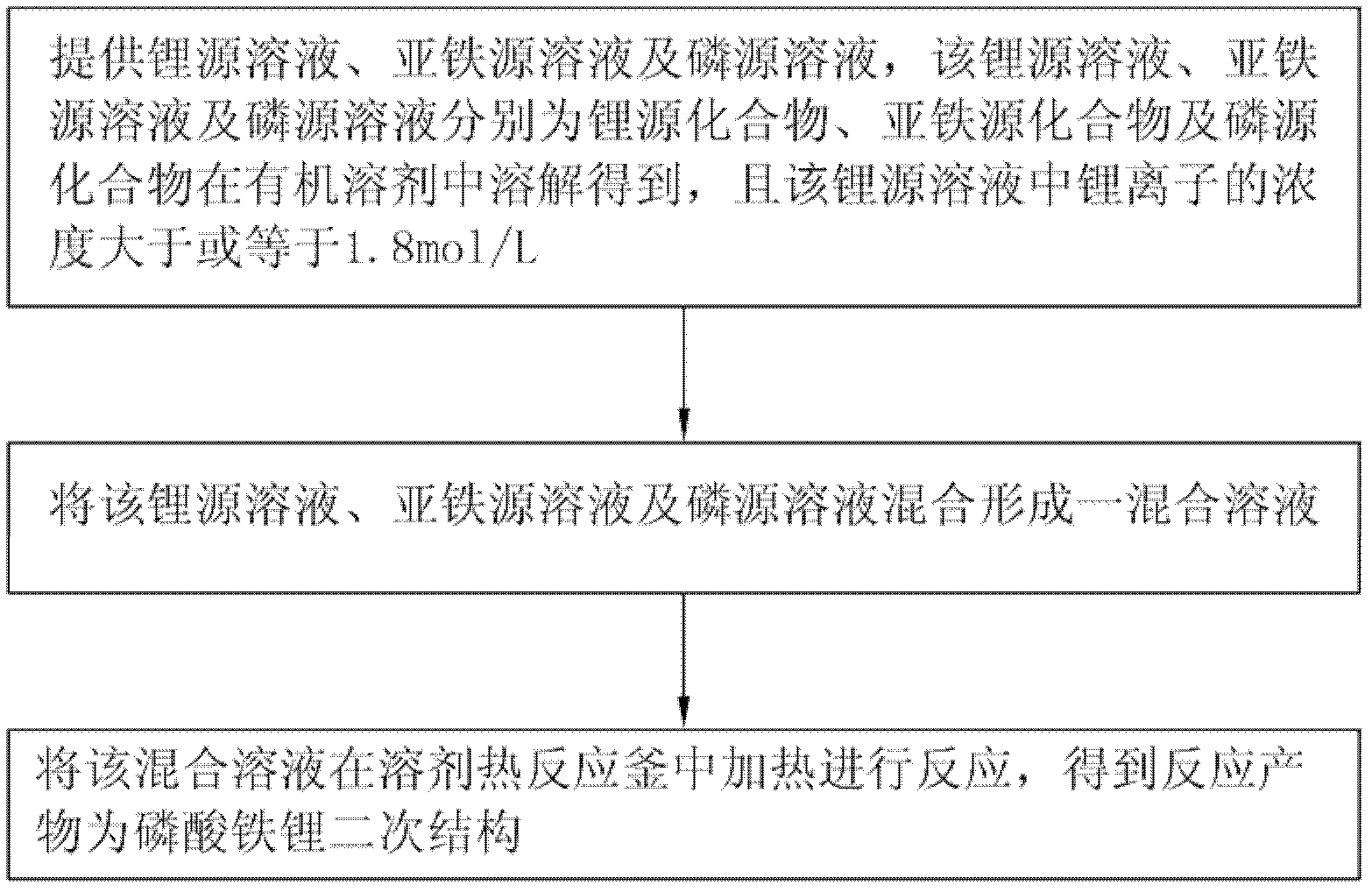
[0026] see figure 1 , the embodiment of the present invention provides a method for preparing a secondary structure of lithium iron phosphate, which includes the following steps:
[0027] Step 1, providing lithium source solution, ferrous source solution and phosphorus source solution respectively, the lithium source solution, ferrous source solution and phosphorus source solution are lithium source compound, ferrous source compound and phosphorus source compound dissolved in an organic solvent respectively Obtained, and the concentration of lithium ions in the lithium source solution is greater than or equal to 1.8mol / L;
[0028] Step 2, mixing the lithium source solution, the ferrous source solution and the phosphorus source solution to form a mixed solution; and
[0029] In step 3, the mixed solution is heated in a solvothermal reaction kettle for reaction, and the reaction product is a secondary structure of lithium iron phosphate.
[0030] In step 1, the lithium source ...
Embodiment 1
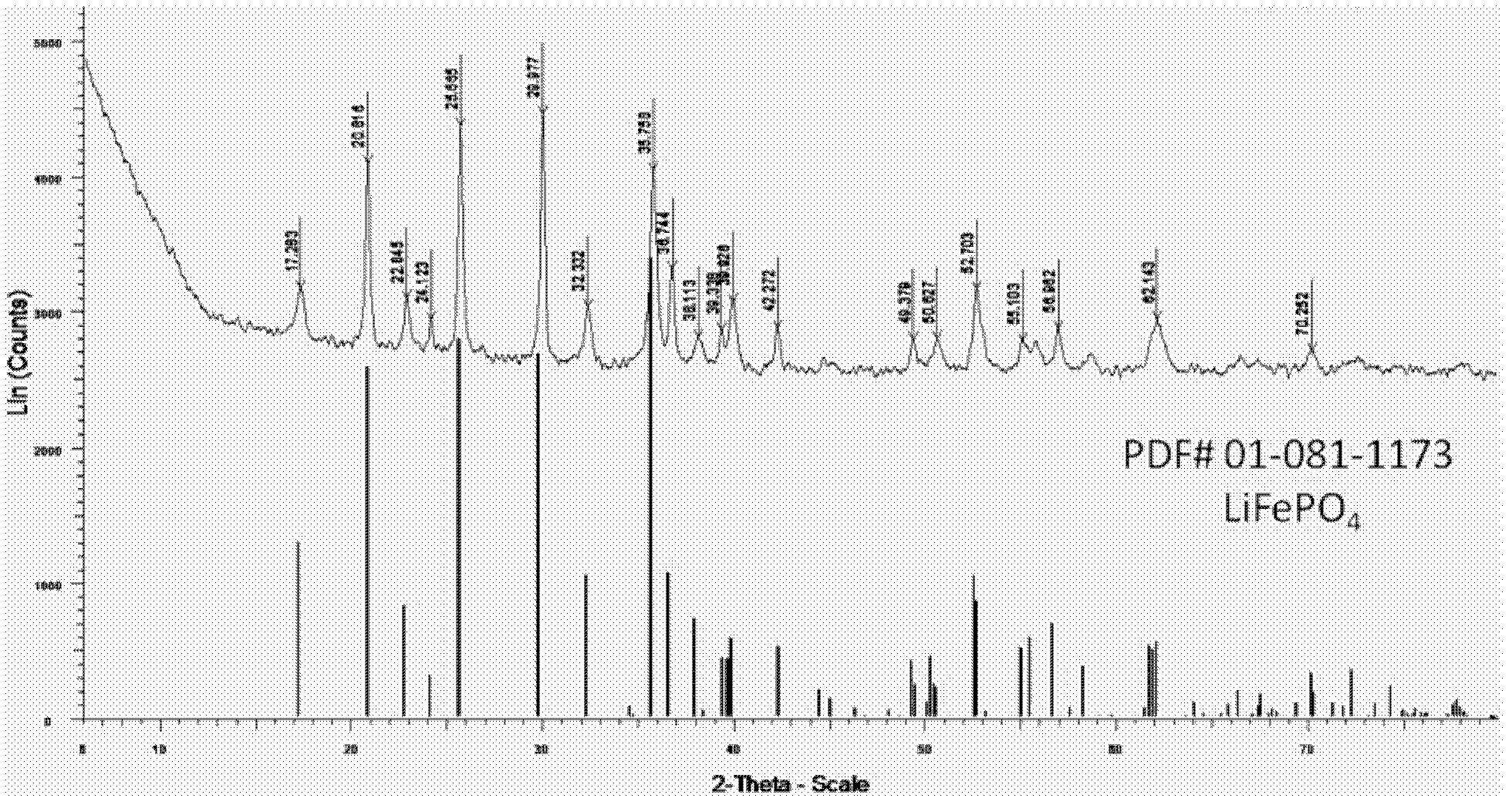
[0054] In this embodiment, the lithium source compound is lithium hydroxide (LiOH·H 2 O), the ferrous source compound is ferrous sulfate (FeSO 4 ·7H 2 O), the phosphorus source compound is phosphoric acid (H 3 PO 4 ), the organic solvent is ethylene glycol. First, 1.6184g LiOH·H 2 O was dissolved in 18.9 mL of ethylene glycol to give Li + Lithium hydroxide solution with a concentration of 2mol / L, at this time LiOH reaches saturation in ethylene glycol. Second, the 3.8922gFeSO 4 ·7H 2 O was dissolved in 23.3 mL of ethylene glycol to give Fe 2+ Ferrous sulfate solution with a concentration of 0.6mol / L. Again, add 944 μL H 3 PO 4 Add it into the ferrous sulfate solution, stir to obtain a mixed solution, then add the lithium hydroxide solution into the above mixed solution, and continue to stir for 30 min. In the finally obtained mixed solution, the molar ratio among the lithium hydroxide, ferrous sulfate and phosphoric acid is 2.7:1:1. Finally, put the mixed solution...
Embodiment 2
[0061] In this embodiment, the lithium source compound is lithium hydroxide (LiOH·H 2 O), the ferrous source compound is ferrous sulfate (FeSO 4 ·7H 2 O), the phosphorus source compound is phosphoric acid (H 3 PO 4 ), the organic solvent is a mixed solvent of ethylene glycol and glycerol volume ratio of 2:1. First, 1.6184g LiOH·H 2 O was dissolved in 18.9 mL of mixed solvent to obtain Li + Lithium hydroxide solution with a concentration of 2 mol / L. Second, the 3.8922gFeSO 4 ·7H 2 O was dissolved in 23.3 mL of mixed solvent to obtain Fe 2+ Ferrous sulfate solution with a concentration of 0.6mol / L. Again, add 944 μL H 3 PO 4 Add it into the ferrous sulfate solution, stir to obtain a mixed solution, then add the lithium hydroxide solution into the above mixed solution, and continue to stir for 30 min. In the finally obtained mixed solution, the molar ratio among the lithium hydroxide, ferrous sulfate and phosphoric acid is 2.7:1:1.5. Put the mixed solution into a sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| apparent density of powders | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com