Method for producing duck tembusu virus inactivated vaccines in large scale
A technology for duck tembusu virus and inactivated vaccine, which is applied in the directions of microorganism-based methods, biochemical equipment and methods, antiviral agents, etc., can solve the problem of low production efficiency, unsuitable for large-scale production of vaccines, and high labor intensity of operation And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of Duck Tembusu Virus Inactivated Vaccine
[0040]Bioreactor: 14L and 40L stirred bioreactor, bioreactor setting parameters are: pH: 7.0, temperature: 36.5°C, dissolved oxygen: 50%, stirring speed: 60r / min;
[0041] Microcarrier: Cytodex1 (manufactured by GE)
[0042] Duck Tambusu virus: WFG36 strain, a strain of Duck Flavivirus (Duck Flavivirus) WFG36 strain, this virus strain has been sent to the Institute of Microbiology, Chinese Academy of Sciences, No. 3, No. 1 Beichen West Road, Chaoyang District, Beijing on April 12, 2011 Preserved by the General Microorganism Center of China Microbiological Culture Collection Management Committee, the number is: CGMCCNo.4718;
[0043] Cell growth medium: DMEM / F12 containing 10% fetal bovine serum (manufactured by GIBCO)
[0044] Virus maintenance solution: DMEM containing 5% fetal bovine serum (manufactured by GIBCO)
[0045] Cell culture:
[0046] (1) Add Cytodex-1 to a 14L bioreactor at a concentration of 15g / L...
Embodiment 2
[0056] Preparation of Duck Tembusu Virus Inactivated Vaccine
[0057] Bioreactor: 14L and 40L stirred bioreactor, bioreactor setting parameters are: pH: 7.0~7.4, temperature: 36.5~37℃, dissolved oxygen: 30~60%, stirring speed: 30~90r / min ;
[0058] Microcarrier: Cytodex1;
[0059] Duck Tembusu virus: WFG36 strain;
[0060] Cell growth medium: DMEM / F12 containing 10% fetal bovine serum ()
[0061] Virus maintenance solution: DMEM containing 5% fetal bovine serum (purchased from GIBCO)
[0062] Cell culture:
[0063] (1) Add Cytodex-1 to a 14L bioreactor at a concentration of 15g / L and sterilize;
[0064] (2) Add BHK cells for culture and observe every day;
[0065] (3) Wait until the cell density of the 14L bioreactor reaches 3.0×10 6 / ml, collect the microcarriers full of cells into a closed container and inoculate into a 40L bioreactor;
[0066] (4) Wait until the cell density of the 40L bioreactor reaches 2.5×10 6 / ml, inoculate duck Tembusu virus WFG36 strain, the ...
Embodiment 3
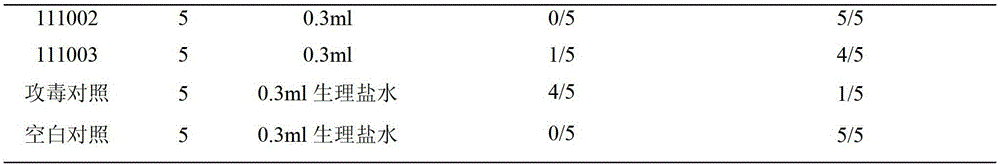
[0074] Inspection of finished product of duck hemorrhagic ovariitis inactivated vaccine
[0075] The vaccine tested in this example is prepared according to the technical scheme provided by the present invention.
[0076] [Properties] The appearance of the vaccines are all milky white emulsions.
[0077] Dosage form oil-in-water type. Take a clean straw, suck a small amount of vaccine and drop it in cold water, except for the first drop, it will not spread.
[0078] Stability Draw 10ml of vaccine into a centrifuge tube, centrifuge at 3000r / min for 15 minutes, no water phase precipitates out at the bottom of the tube.
[0079] Viscosity Use a 1ml straw (the inner diameter of the lower mouth is 1.2mm, and the inner diameter of the upper mouth is 2.7mm) to draw 1.0ml of vaccine at about 25°C, let it flow out vertically and naturally, and record the time required for the outflow of 0.4ml, all within 8 seconds.
[0080] [Sterility test] Carried out according to the method stipul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com