A kind of preparation method of PDPT fuel cell catalyst for direct formic acid fuel cell
A formic acid fuel cell and fuel cell technology, which is applied in battery electrodes, chemical instruments and methods, catalysts for physical/chemical processes, etc., can solve problems such as synthesis and production of catalysts that are difficult to apply, and achieve the effects of simple operation and expansion of synthesis scale.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
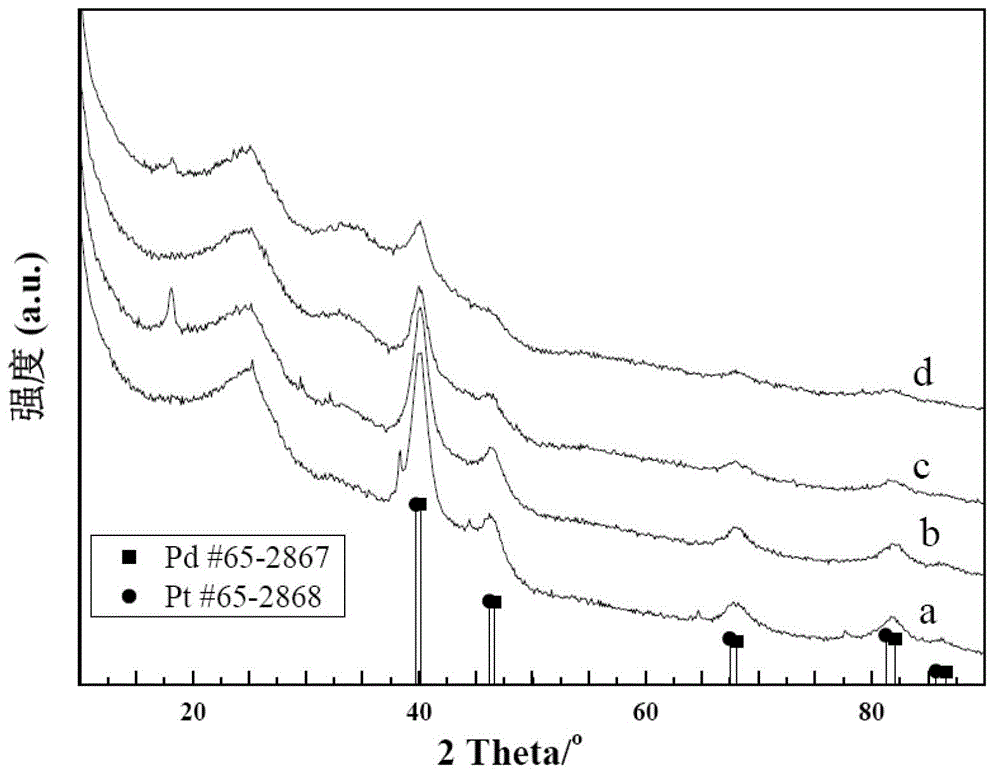
[0045] Firstly, Pd / C with a Pd content of 20wt% was prepared, and the US CABOT carrier Vulcan XC-72R was used. The parameters of the carrier are:
[0046] Table 1 Parameters of the carrier
[0047] model
VXC-72
powdery
specific surface area
254
DBP oil absorption value (cc / 100g)
192
Particle size (nm)
30
Tinting strength (ASTM)
87
Density (g / l)
96.1
[0048] First, 80 mg of the carrier was dispersed in 200 mL of deionized water by ultrasonication for 0.5 h. Then add the precursor PdCl 2 aq (4mg Pd / mL, HCl 5M), again by 0.5h ultrasonic dispersion. Use concentrated NaOH solution to adjust its pH to 10, add NaBH dropwise 4 Solution (NaBH 4 80mg / 50mL of deionized water) to reduce the precursor. After the reduction is completed, a replacement step is performed: adding H to the above-mentioned reduced Pd precursor 2 PtCl 6 Solution (18.105mg Pt / mL) and KCl 0.045g, stirred...
Embodiment 2
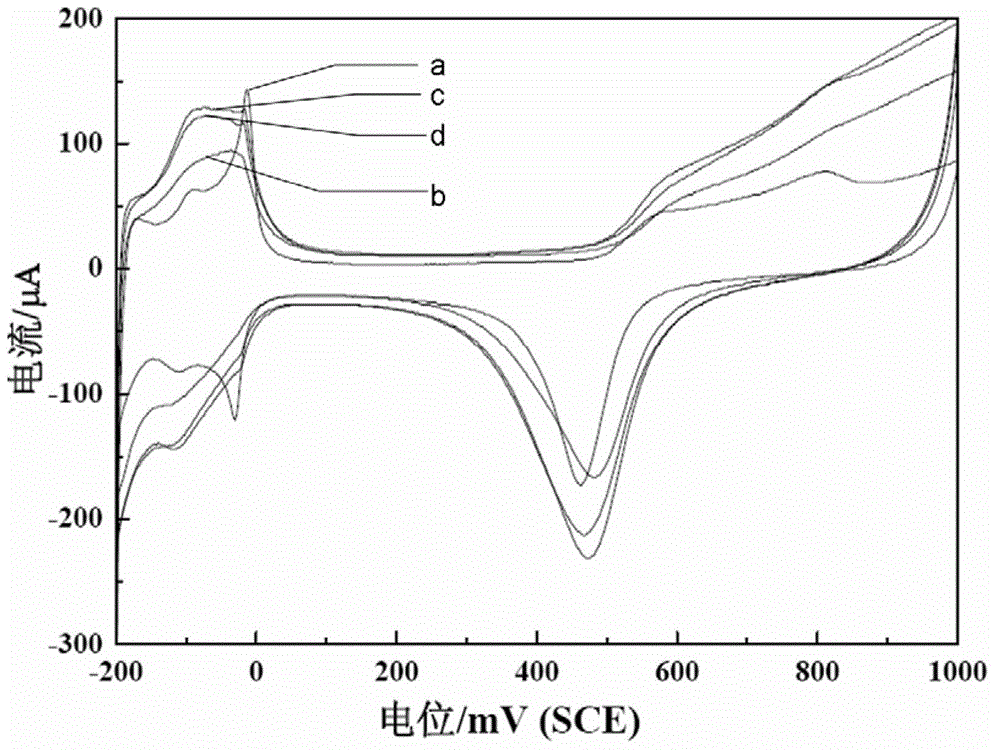
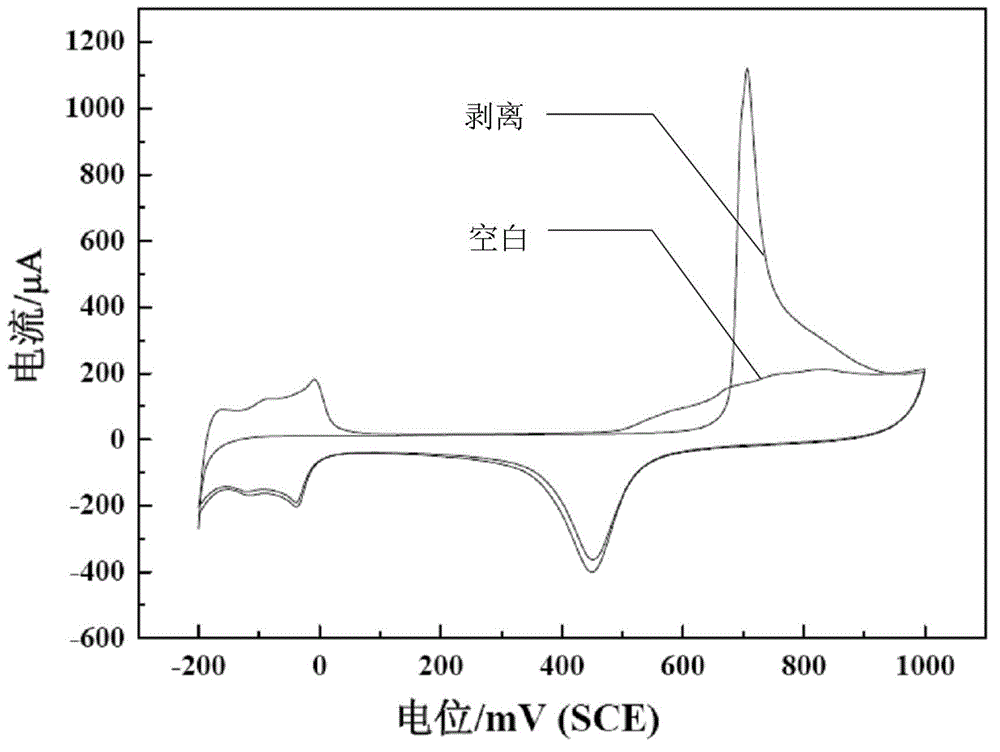
[0054] Example 2: The sample preparation method is basically the same as in Example 1, except that KCl is replaced by 0.060 g of KBr. Test method is identical with embodiment 1, 0.5M H 2 SO 4 The cyclic voltammetry curve in solution is as follows figure 2 , CO adsorption and desorption experiments such as Figure 6 , 0.5M H 2 SO 4 The cyclic voltammetry test and chronoamperometry test results carried out with 0.5M HCOOH solution are shown in Figure 7 and Figure 8 .
Embodiment 3
[0055] Example 3: The sample preparation method is basically the same as that of Example 1, except that no KCl is added. Test method is identical with embodiment 1, 0.5M H 2 SO 4 The cyclic voltammetry curve in solution is as follows figure 2 , CO adsorption and desorption experiments such as Figure 4 , 0.5M H 2 SO 4 The cyclic voltammetry test and chronoamperometry test results carried out with 0.5M HCOOH solution are shown in Figure 7 and Figure 8 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| tinctorial strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com