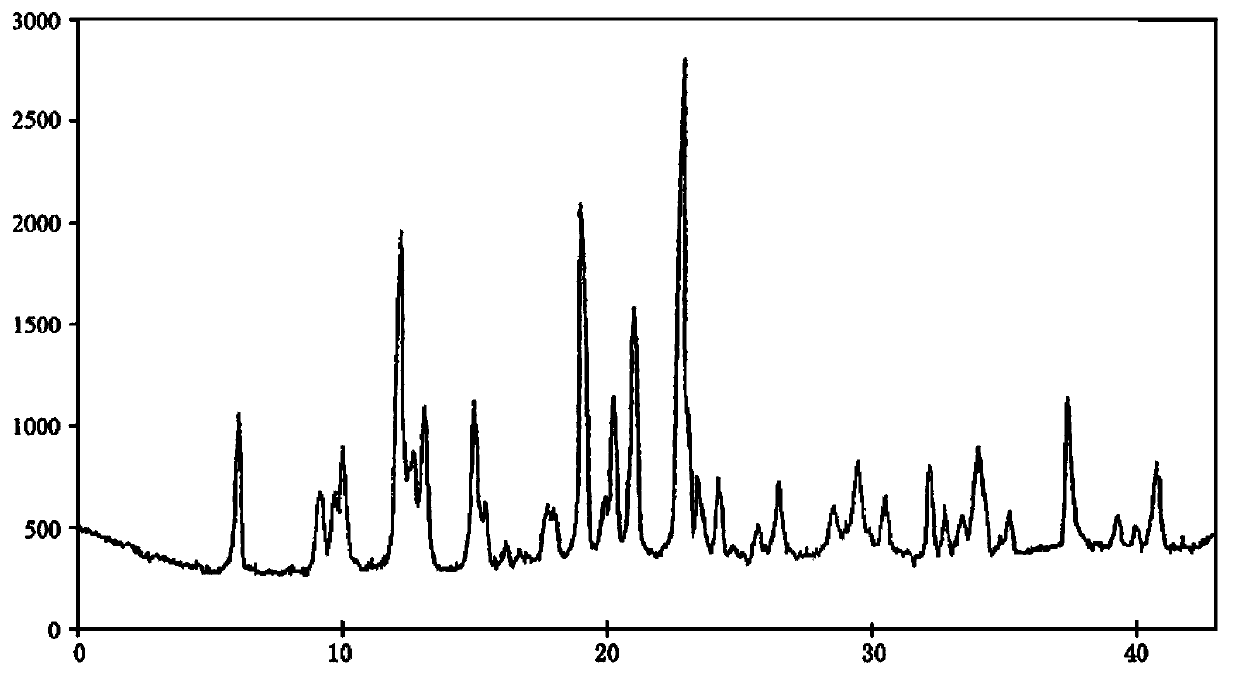
Sodium pantothenate compound, and composition preparation containing it
A technology of sodium pantothenate and compound, applied in the field of sodium pantothenate compound and composition preparation containing the compound, can solve the problems of inability to guarantee the stability of the preparation, the crystal quality is not stable enough, the preparation process is unstable, etc. Medication safety and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 sodium pantothenate compound
[0044] (1) Take the commercially available crude sodium pantothenate, add water equivalent to 40 times the weight of the crude sodium pantothenate, stir and dissolve at 50°C, and concentrate under reduced pressure to 1 / 5 of the original volume; wherein, concentrate under reduced pressure under vacuum at 60 Below ℃, the vacuum degree is 0.07 MPa;
[0045] (2) Add activated carbon 0.08 times the weight of crude sodium pantothenate to the solution for decolorization, and filter;
[0046] (3) Stir and add ethanol:ether mixed solution whose volume is 1 / 4 of the solution to the filtrate; the volume of ethanol and ether in the ethanol:ether mixed solution is 1:8. The speed of / min cools the filtrate to 12°C; wherein, the stirring speed is 8rmp;
[0047] (4) Stop stirring, cool the solution down to 2°C at a uniform speed within 30 minutes, and place it under an ultrasonic field to grow crystals for 8 hours, the ul...
Embodiment 2
[0049] The preparation of embodiment 2 sodium pantothenate compound
[0050] (1) Take the commercially available crude sodium pantothenate, add water equivalent to 30 times the weight of the crude sodium pantothenate, stir and dissolve at 45°C, and concentrate under reduced pressure to 1 / 4 of the original volume; wherein, concentrate under reduced pressure under vacuum at 60 Below ℃, the vacuum degree is 0.06 MPa;
[0051] (2) Add activated carbon 0.05 times the weight of crude sodium pantothenate to the solution for decolorization, and filter;
[0052] (3) Stir and add ethanol:ether mixed solution whose volume is 1 / 5 of the solution to the filtrate; the volume of ethanol and ether in the ethanol:ether mixed solution is 1:5. The speed of / min cools the filtrate to 10°C; wherein, the stirring speed is 6rmp;
[0053] (4) Stop stirring, cool the solution down to 0°C within 20 minutes, and place it in an ultrasonic field to grow crystals for 6 hours, the ultrasonic frequency is ...
Embodiment 3
[0054] The preparation of embodiment 3 sodium pantothenate compound
[0055] (1) Take the commercially available crude sodium pantothenate, add water equivalent to 45 times the weight of the crude sodium pantothenate, stir and dissolve at 55°C, and concentrate under reduced pressure to 1 / 6 of the original volume; wherein, concentrate under reduced pressure under vacuum at 60 Below ℃, the vacuum degree is 0.08 MPa;
[0056] (2) Add activated carbon 0.1 times the weight of the crude product of sodium pantothenate to the solution for decolorization, and filter;
[0057] (3) Stir and add ethanol:ether mixed solution whose volume is 1 / 3 of the solution to the filtrate; the volume of ethanol and ether in the ethanol:ether mixed solution is 1:12 while feeding at 2.5°C / The speed of min will cool the filtrate to 15°C; wherein, the stirring speed is 12rmp;
[0058] (4) Stop stirring, cool the solution down to 5°C within 40 minutes, and place it under an ultrasonic field to grow cryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com