Sulfur-containing epoxide resin and preparation method thereof
A technology of sulfur epoxy resin and epoxy resin, which is applied in the field of sulfur-containing epoxy resin and its preparation, can solve problems such as difficult large-scale application, shortened product storage period, and easy entanglement of molecular chains, and achieve gel The effect of shortening time, short reaction time and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
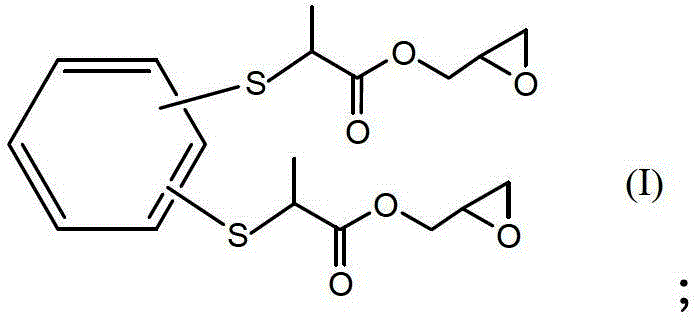
[0025] Under the condition of 15-20°C, slowly add 0.1mol dimercapto m-benzene, 0.2mol glycidyl methacrylate, and 0.5% hydroquinone of the mass of glycidyl methacrylate in sequence, mix well and wait for 5-10 After stirring and reacting for 10 hours at 140°C, the unreacted glycidyl methacrylate was removed at 140°C under a vacuum of 2mmHg to obtain a light yellow liquid, which is the sulfur-containing epoxy resin. The number average molecular weight was 426g / mol, the viscosity at 25°C is 650cp, and the epoxy value is 0.47mol / 100g.
[0026] The structural formula of sulfur-containing epoxy resin is as follows:
[0027]
Embodiment 2
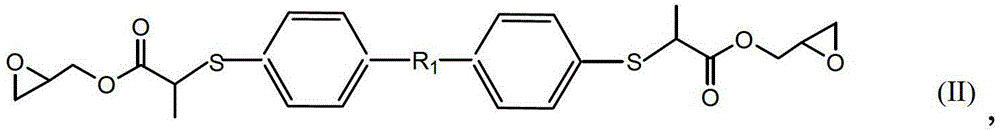
[0029] Under the condition of 10-15°C, slowly add 0.1mol dimercapto-p-benzene, 0.2mol glycidyl methacrylate, and 0.5% hydroquinone of the mass of glycidyl methacrylate in sequence, mix well and stir for 10- After reacting at 15°C for 8 hours, then remove unreacted glycidyl methacrylate at 140°C under a vacuum of 2 mmHg to obtain a light yellow liquid sulfur-containing epoxy resin. The number average molecular weight was 426g / mol, 25 The viscosity at ℃ is 600cp, and the epoxy value is 0.46mol / 100g.
[0030] The structural formula of sulfur-containing epoxy resin is as follows:
[0031]
Embodiment 3
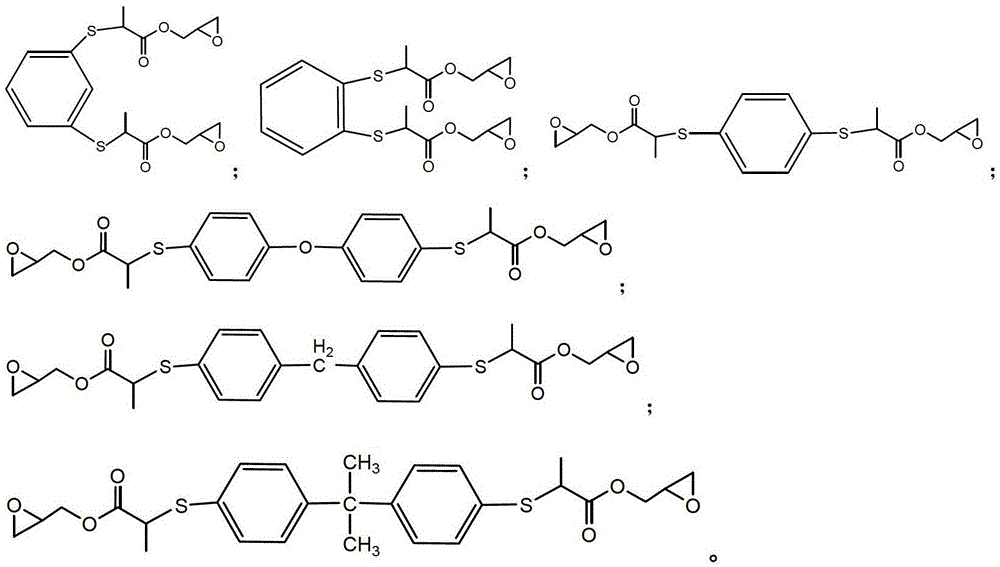
[0033] Under the condition of 15-20°C, slowly add 0.1mol dimercapto o-phthalene, 0.2mol glycidyl methacrylate, and 0.5% hydroquinone of the mass of glycidyl methacrylate in sequence, mix well and then stir for 5- After reacting at 10°C for 4 hours, then remove unreacted glycidyl methacrylate at 140°C under a vacuum of 2mmHg to obtain a light yellow liquid sulfur-containing epoxy resin. The number average molecular weight was 426g / mol, 25 The viscosity at ℃ is 500cp, and the epoxy value is 0.47mol / 100g.
[0034] The structural formula of sulfur-containing epoxy resin is as follows:
[0035]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com