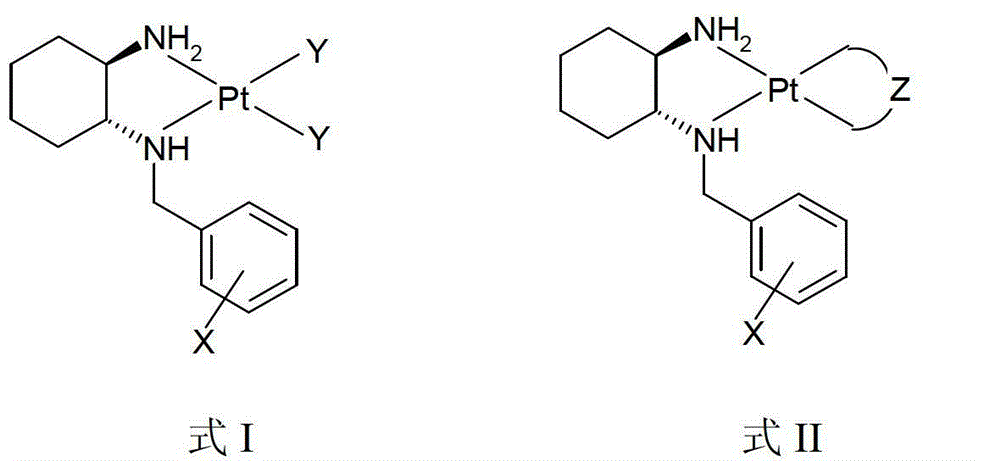
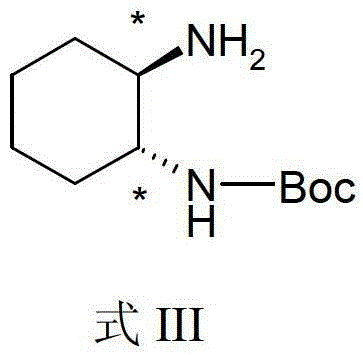
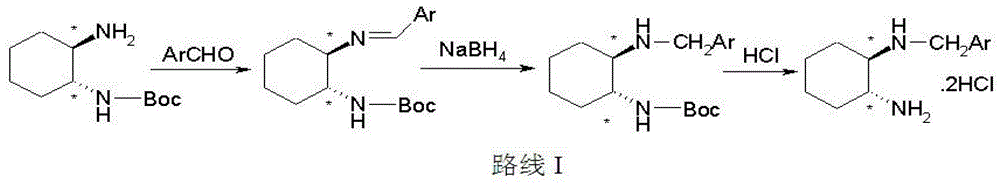
Divalent-platinum complex containing sterically-hindered aryl group and preparation method and application thereof
A technology of platinum complexes and groups, applied in the field of anti-tumor platinum complexes, can solve the problems of toxicity, limited application of platinum drugs, drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1. complex 1 (molecular formula: C 13 h 20 Cl 2 N 2 Pt)
[0034]
[0035] Potassium tetrachloroplatinate (5.00mmol) and ligand (N-benzyl-1R, 2R-cyclohexanediamine, 5.00mmol) were mixed in 80mL of water, stirred at 30°C for 24 hours in the dark, a large amount of yellow precipitate Generated, filtered, washed repeatedly with water, washed with a small amount of ethanol, dried to obtain yellow powder, yield: 86%. IR(KBr,cm -1 ):3466(br),3120,2935,2860,1580,1452,751,702; 1 H-NMR (d 6 -DMSO / TMS,ppm):δ1.09-2.01(m,8H,CH 2 of DACH),δ2.12-2.25(m,2H,NHCH and NH 2 CH),δ3.91-4.54(m,2H,NHCH 2Ph),δ6.11-6.16(dd,2H,CHNH 2 ),δ7.29-7.31(m,1H,CH 2 NH), δ7.38-8.11(m, 5H, Ar-H); ESI-MS: m / z[M-Cl] + =435(100%).
Embodiment 2
[0038] Embodiment 2. The preparation of complex 2 (molecular formula: C 13 h 19 Cl 2 FN 2 Pt)
[0039]
[0040] Prepared with reference to the method described in Example 1, the ligand is N-(2-fluorobenzyl)-1R,2R-cyclohexanediamine, the reaction temperature is 50°C, and it is stirred for 24 hours in the dark to obtain a light yellow powder. The yield is: 97%. IR(KBr,cm -1 ):3138(br),2933,2862,1579,1494,1451,1229,1187,1139,763; 1 H-NMR (d 6 -DMSO / TMS,ppm):δ0.80-1.85(m,8H,CH 2 of DACH),δ2.11-2.23(m,2H,NHCH and NH 2 CH),δ3.89-4.40(m,2H,NHCH 2 Ph),δ4.96-5.47(dd,2H,CHNH 2 ), δ6.75-7.45(m, 4H, Ar-H), δ8.89-8.93(m, 1H, CH 2 NH); ESI-MS: m / z [M-Cl] + =453(100%).
Embodiment 3
[0041] The preparation of embodiment 3. complex 3 (molecular formula: C 13 h 19 Cl 2 FN 2 Pt)
[0042]
[0043] Prepared with reference to the method described in Example 1, the ligand is N-(3-fluorobenzyl)-1R,2R-cyclohexanediamine, the reaction temperature is 60°C, and it is stirred for 12 hours in the dark to obtain a light yellow powder. The yield is: 85%. IR(KBr,cm -1 ):3267(br),3188,3100,2938,1587,1451,1258,1147,791,753; 1 H NMR (d 6 -DMSO / TMS,ppm):δ0.76-1.98(m,8H,CH 2 of DACH),δ1.98-2.23(m,2H,NHCH and NH 2 CH),δ3.67-4.58(m,2H,NHCH2Ph),δ5.01-5.48(dd,2H,CHNH 2 ), δ6.72-7.86(m, 4H, Ar-H), δ8.25-8.27(m, 1H, CH 2 NH); ESI-MS: m / z [M-Cl] + =453(100%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com