Method of avoiding phenomenon of accelerating blood clearance by continuously and repeatedly injecting polyethylene glycol (PEG) lipidosome of epirubicin hydrochloride
A kind of epirubicin hydrochloride, a technology for accelerating blood clearance, applied in the field of tissue distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Preparation of epirubicin hydrochloride liposomes
[0066] The prescription is:
[0067] HSPC 3.0g
[0068] CH 1.0g
[0069] MPEG 2000 -DSPE 1.0 g
[0070] The preparation process is:
[0071] At 65 °C, dissolve the prescribed amount of membrane material with 5% ethanol (v / v), inject 60 mL of hydration medium (200 mmol L -1 (NH 4 ) 2 SO 4 solution), incubated for 20 min to obtain the primary liposome, which was treated by micro-fluidization (pressure of 14000 psi) to reduce the particle size to 100 nm, and passed through microporous membranes of 0.8, 0.45 and 0.22 μm in sequence, namely Obtain blank liposomes.
[0072] Gradient establishment: take blank liposomes, mix with mixed resin (the mixing ratio of 732 sodium cation exchange resin and 717 chloride anion exchange resin is 1:2 according to the wet volume) and let it stand for 5 minutes, then centrifuge at 2000 rpm for 4 minutes , to build the gradient.
[0073] Drug loading: add epirubicin hy...
Embodiment 2
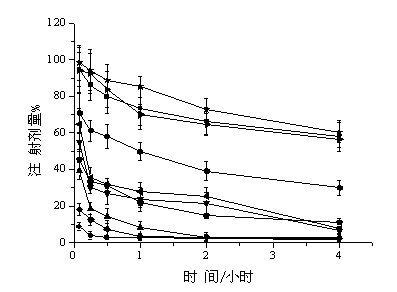
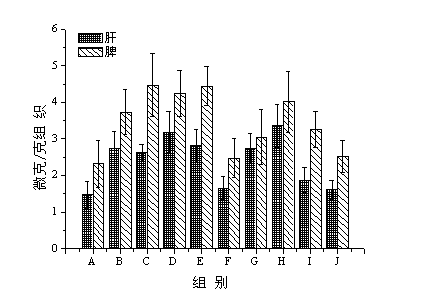
[0074] Example 2 Effect of the first injection of blank liposomes and epirubicin hydrochloride liposomes on the ABC phenomenon of the second injection of epirubicin hydrochloride liposomes
[0075] 1 Processing of plasma and tissue samples
[0076] 1.1 Processing of plasma samples: Take 0.1 mL of rat plasma, put it in a 7 mL tube, add 0.3 mol·L -1 HCl methanol-water (50:50, v / v) solution 4.9 mL, vortex 30 s to mix. The suspension was centrifuged at 10,000 rpm for 10 min, and the supernatant was taken to measure the fluorescence value.
[0077] 1.2 Treatment of tissue samples: Take 0.5 g of tissue in a 7 mLEp tube, add 1 mL of normal saline for high-speed dispersion and homogenate, pipette 0.2 mL of tissue homogenate precisely, add 0.3 mol·L -1 HCl methanol-water (50:50, v / v) solution 4.8 mL, vortex 30 s to mix. The suspension was centrifuged at 10,000 rpm for 10 min, and the supernatant was taken to measure the fluorescence value.
[0078] 2 Establishment of in vivo ...
Embodiment 3
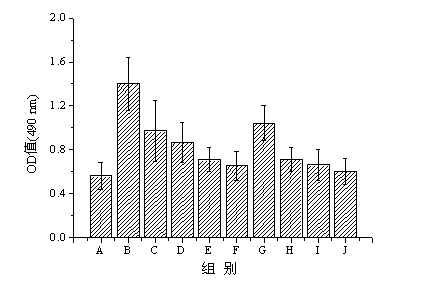
[0089] Example 3 Determination of IgM content of anti-PEG in serum
[0090] Refer to the method of Ichihara et al. (Ichihara M, Shimizu T, et al. Anti-PEG IgM Response against PEGylated Liposomes in Mice and Rats[J]. Pharmaceutics, 2011, 3: 1–11) to determine the content of anti-PEG IgM in serum Determination. The specific process is as follows: the MPEG 2000 -DSPE was prepared with absolute ethanol to a concentration of 0.2 mmol L -1 solution, take 50 μL and add it to a 96-well plate. After the 96-well plate is completely dry at room temperature, add Tris buffer containing 1% BSA (50 mmol L -1 Tris, 0.14 mmol L -1 NaCl, pH 8.0) 100 μL for blocking for 1 h, and then successively washed three times with Tris buffer containing 0.05% Tween20. Serum samples were diluted 100 times, 100 μL was added to each well, incubated at room temperature for 1 h, washed five times with Tris buffer containing 0.05% Tween20, and 1 μg / mL horseradish peroxidase-linked goat antibody was adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com