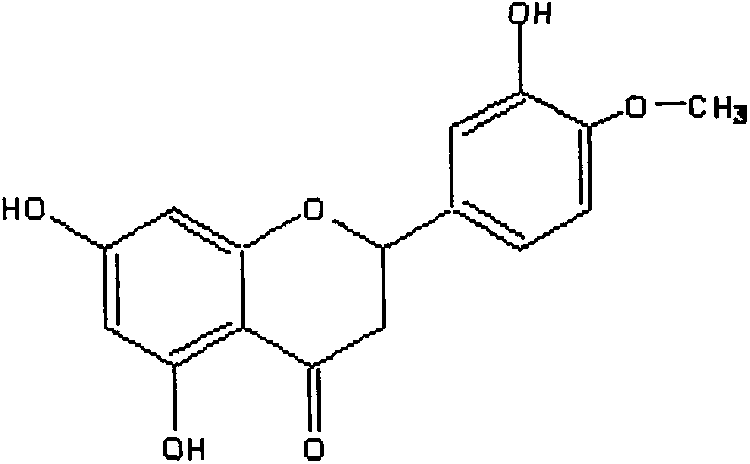
Clean preparation process of high-purity hesperetin
A preparation process, the technology of hesperetin, which is applied in the field of clean preparation process of high-purity hesperetin, can solve the problems of high cost, unsuitable for industrial production, complicated steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] Close the mouth of the reaction kettle, vacuum pump 1300kg of 99% methanol with accurate measurement into the reaction kettle, open the mouth of the reaction kettle, start stirring, adjust the pH of the methanol solution to 1-2 with concentrated sulfuric acid, add 1.0-1.5% vitamin C, and slowly add it from the mouth of the reaction kettle Hesperidin 200.0kg, seal the reaction kettle mouth, open the heat-carrying steam valve of the reaction kettle to heat, close the heat-carrying steam valve when the pressure indicated by the pressure gauge on the reaction kettle reaches 0.22MPa, start timing when the pressure rises to 0.25MPa, and maintain the pressure in the kettle At 0.22~0.25Mpa5~7h. After the reaction was complete, the pressure was lowered to obtain a clear and translucent hesperetin reaction solution. The reaction solution was concentrated under reduced pressure at 50°C, and the concentration was stopped after the alcohol content of the concentrated solution reache...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com