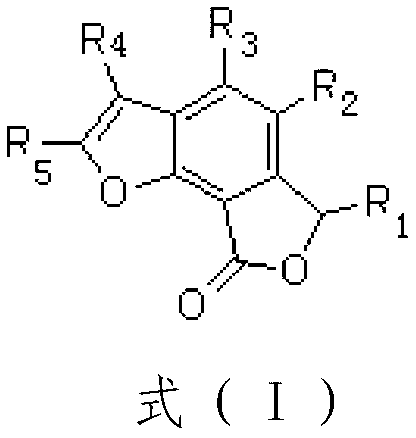
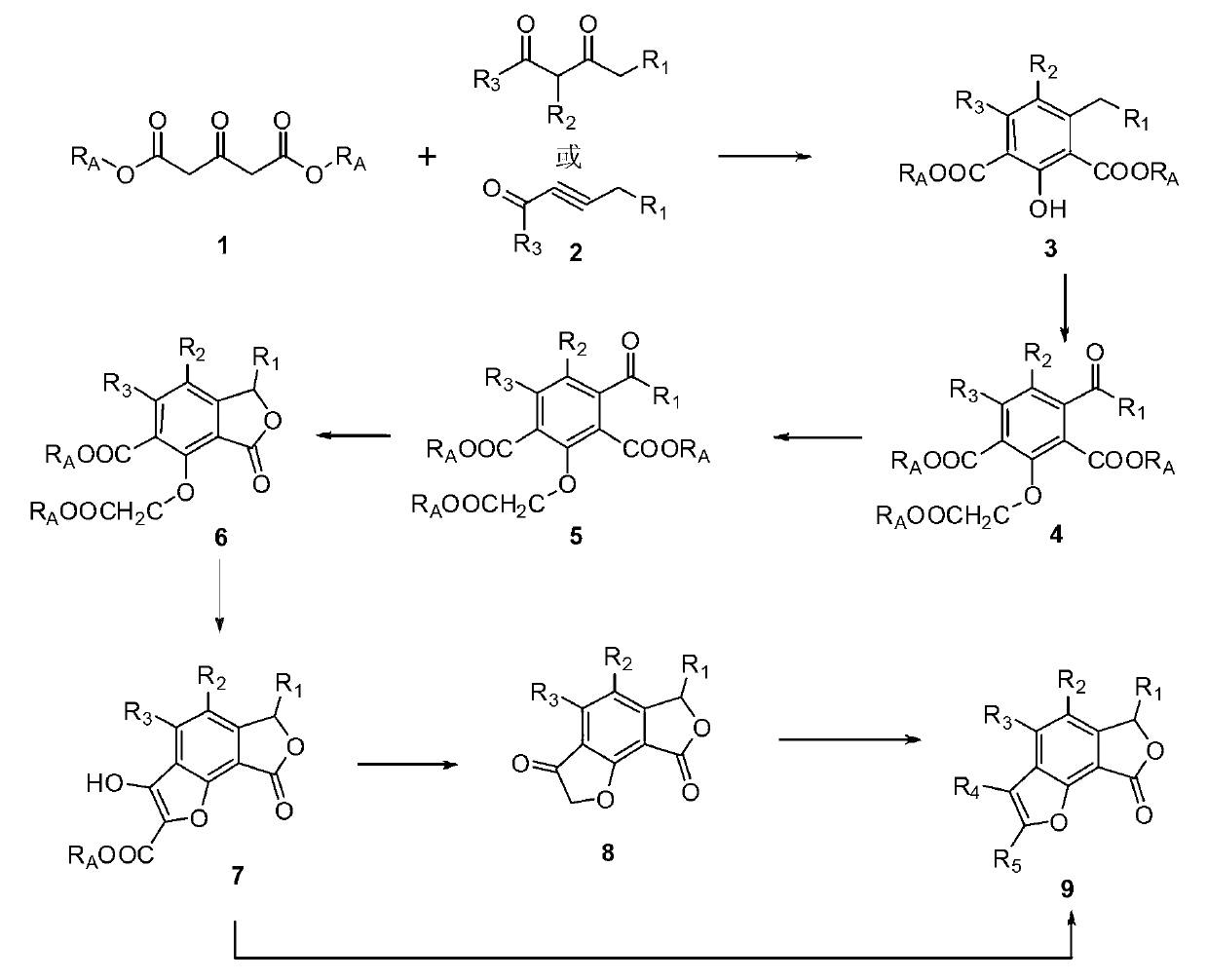
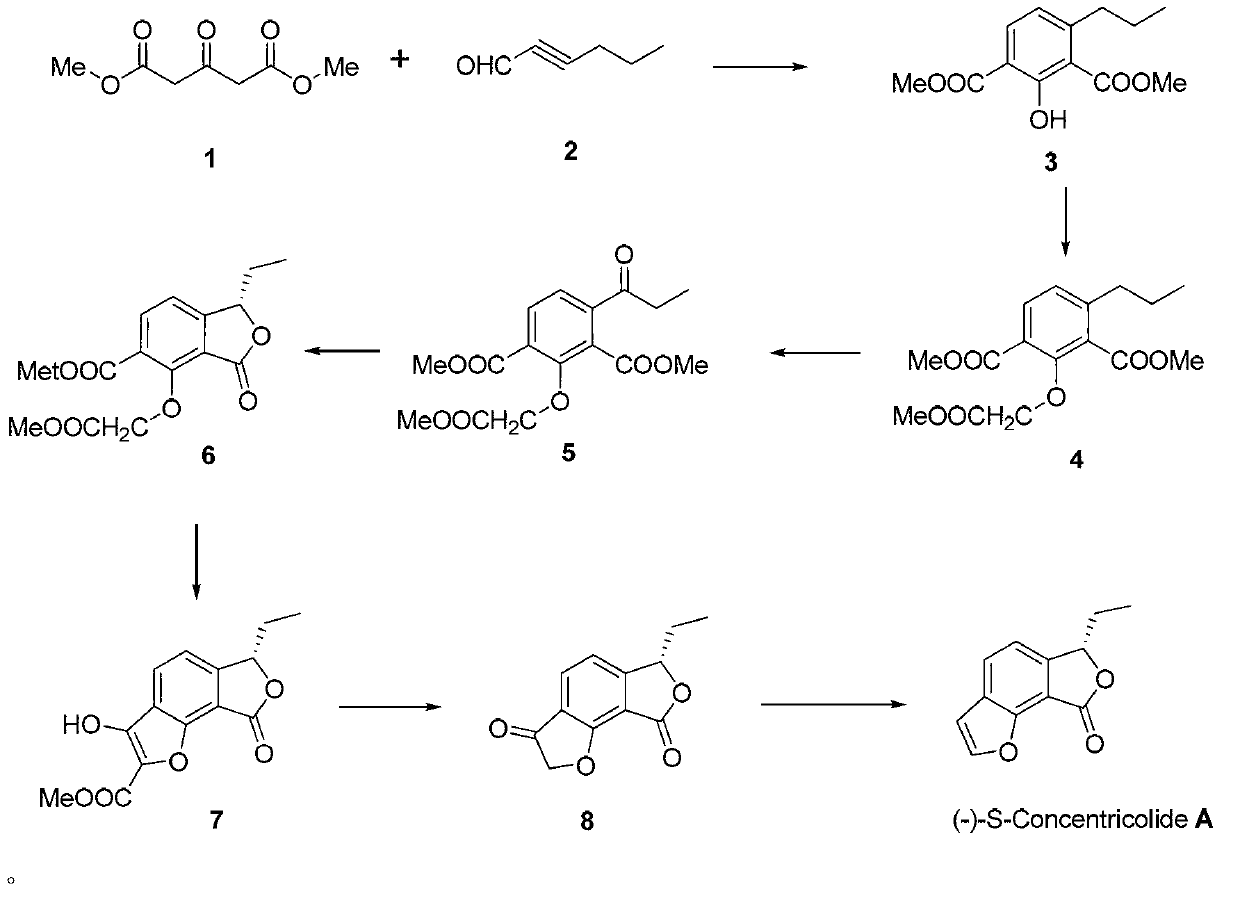
Total synthesis method of natural active product concentricolide and its analogue
A carboncoccus, total synthesis technology, applied in organic chemistry and other directions, can solve the problems of harsh synthesis conditions, inability to prepare in large quantities, and low yield, and achieve the effect of good general adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Preparation of compound 3
[0044]
[0045] Under argon protection, take 80mg (2mmol, 60%) NaH in a 50mL dry three-necked flask, add 5mL of anhydrous THF, then add 278mg (1.60mmol) dimethyl 1,3-acetonedicarboxylate 1, and 170mg Alkynal 2, reacted at room temperature for 4 hours, the raw material disappeared, and the reaction was stopped. Evaporate THF, dissolve the residue with 8 mL of ethyl acetate, wash with 1N hydrochloric acid and distilled water successively, anhydrous Na 2 SO 4 After drying, the solvent was concentrated and separated by column chromatography to obtain 181 mg of light yellow oily substance 3 with a yield of 54%.
[0046] Compound 3 1 H-NMR and 13 C-NMR data:
[0047] 1 H-NMR (400MHz, CDCl3) δppm: 0.94 (3H, t, J = 7.2Hz), 1.56-1.67 (2H, m), 2.58 (2H, t, J = 8.0Hz), 3.94 (6H, m), 6.76(1H,d,J=8.0Hz),7.79(1H,d,J=8.0Hz),11.12(1H,s).
[0048] 13 C-NMR (100MHz, CDCl3) δppm: 14.1, 24.1, 36.2, 52.5, 52.6, 110.6, 120.4, 123.2, 130.9, 148.8, 1...
Embodiment 2
[0086] Compared with Example 1, the only difference in this Example 1 is that Steps 4-7 are different. In this Example, when reducing the carbonyl group of Compound 5, sodium borohydride was used to reduce the racemic compound, see Step 8 for details. -11.
[0087] (8) Preparation of Compound 10
[0088]
[0089] Add compound 542mg to dissolve in 1mL EtOH in 50mL one-mouth bottle, add 6mg (1.57mmol) NaBH 4 , stirred at room temperature for 12 h, TLC detected that the reaction of the raw materials was complete, 2 mL of dilute hydrochloric acid was added to quench the reaction, extracted three times with ethyl acetate (3×5 mL), and dried over anhydrous sodium sulfate. After filtration, the solvent was concentrated and separated by column chromatography to obtain 28 mg of compound 6 with a yield of 65%.
[0090] (9) Preparation of Compound 11
[0091]
[0092] Use the same operation as step 5.
[0093] (10) Preparation of Compound 12
[0094]
[0095] Use the same o...
Embodiment 3-4
[0101] Compared with Example 1, the only difference in this example is that steps 6-7 are different. In this example, other reagents are used to modify the furan ring of compound 5 or compound 6, thereby synthesizing the compound with 6-hydrogen-1,7 -Carbonococcin analogues 13 and 14 of the dioxa[E]inden-8-one core.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com