A kind of preparation method of tranexamic acid
A technology of tranexamic acid and aminomethylcyclohexanecarboxylic acid, which is applied in the field of preparation of tranexamic acid, can solve the problem that the total yield is only 14%, and achieve reduced raw material consumption, high yield, and reduced operation The effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
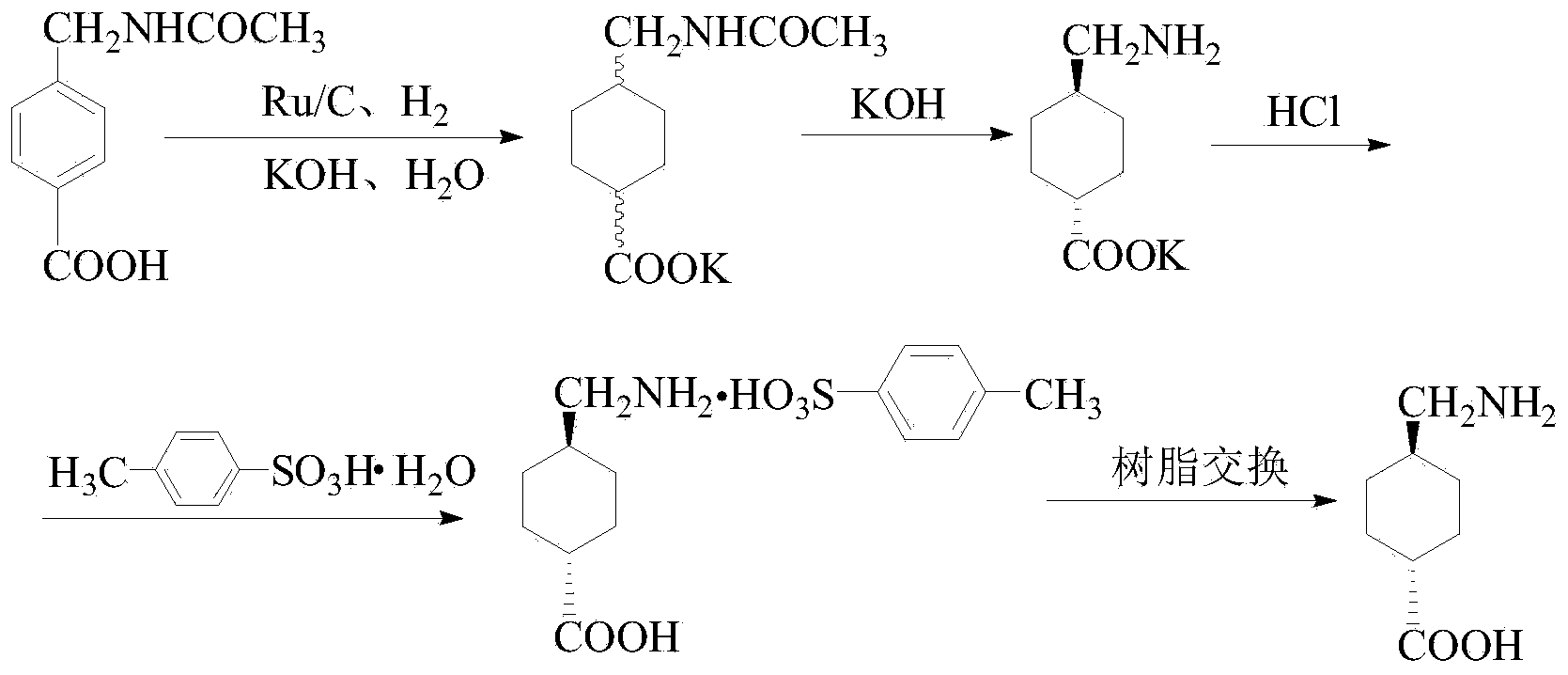
[0034] 1. Catalytic hydrogenation
[0035] In a 2L autoclave, add 234g 4-(acetamidomethyl)benzoic acid, 1000mL deionized water, 50.9g NaOH, 23g Raney nickel, N 2 Substitution, hydrogen replacement, under the conditions of 100°C and 6MPa, react until there is no pressure drop, and then react for 3h. Cool down to room temperature, remove all remaining hydrogen, take it out with a suction filter bottle, filter, remove the catalyst, rinse the catalyst with water to obtain a cis-trans mixture of potassium salt of 4-(acetylaminomethyl)cyclohexylformate.
[0036] 2. Deprotection transformation into salt
[0037] Add 97.0g NaOH to the 4-(acetylaminomethyl) cyclohexyl formic acid potassium salt cis-trans mixture, stir to dissolve, pass N 2 , distill water at 130°C until there is almost no distillate, heat up to 200°C in an oil bath, the reaction solution gradually solidifies and dries, and continues to bake at this temperature for 16 hours to obtain a dry solid. Cool down to room te...
Embodiment 2
[0041] 1. Catalytic hydrogenation
[0042] In a 2L autoclave, add 234g 4-(acetamidomethyl)benzoic acid, 1000mL deionized water, 50.9g NaOH, 23g Raney nickel, N 2 Substitution, hydrogen replacement, under the conditions of 180°C and 15MPa, react until there is no pressure drop, and then react for 3h. Cool down to room temperature, remove all remaining hydrogen, take it out with a suction filter bottle, filter, remove the catalyst, rinse the catalyst with water to obtain a cis-trans mixture of potassium salt of 4-(acetylaminomethyl)cyclohexylformate.
[0043] 2. Deprotection transformation into salt
[0044] Add 97.0g NaOH to the 4-(acetylaminomethyl) cyclohexyl formic acid potassium salt cis-trans mixture, stir to dissolve, pass N 2 , distill water at 150°C until there is almost no distillate, heat up to 280°C in an oil bath, the reaction solution gradually solidifies and dries, and continues to bake at this temperature for 24 hours to obtain a dry solid. Cool down to room t...
Embodiment 3
[0048] 1. Hydrogenation
[0049] 2L autoclave, add 234g 4-(acetamidomethyl)benzoic acid, 1000mL deionized water, 61g NaOH, 25g Raney nickel, N 2Substitution, hydrogen replacement, at 150°C, 12MPa, react until there is no pressure drop, and then react for 3h. Cool down to room temperature, remove all remaining hydrogen, take it out with a suction filter bottle, filter, remove the catalyst, rinse the catalyst with water to obtain a cis-trans mixture of potassium salt of 4-(acetylaminomethyl)cyclohexylformate.
[0050] 2. Deprotection transformation into salt (best)
[0051] In 4-(acetamidomethyl) cyclohexyl formic acid potassium salt cis-trans mixture, add 83g NaOH, stir to dissolve, logical N 2 , Distill water at 130°C until there is almost no distillate, heat up to an oil bath at 230°C, the reaction solution gradually solidifies and dries, and continues to bake at this temperature for 18 hours to obtain a dry solid. Cool down to room temperature and add 1600mL of water, sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com